Fentanyl drug profile

About fentanyl
Fentanyl is a narcotic analgesic with a potency at least 80 times that of morphine. Fentanyl and its derivatives (Alfentanil, Sufentanil, Remifentanil and Carfentanil) are used as anaesthetics and analgesics in both human and veterinary medicine (Carfentanil). They are subject to international control as are a range of highly potent non-pharmaceutical fentanyl (NPF) derivatives, such as 3-methylfentanyl, synthesised illicitly and sold as ‘synthetic heroin’, or mixed with heroin.
Chemistry
Fentanyl (CAS-437-38-7) is a piperidine derivative. The fully systematic name (IUPAC) is N-(1-(2-phenethyl)-4-piperidinyl-N-phenyl-propanamide.
Molecular structure
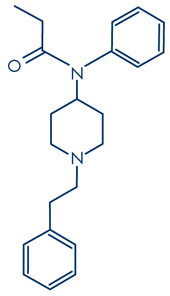
Molecular formula: C22H28N2O
Molecular weight: 336.471 g/mol
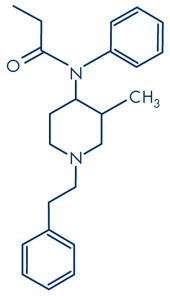
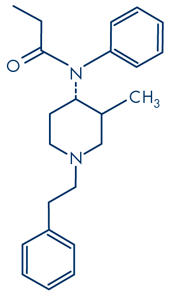
Different fentanyl derivatives have been developed by the legitimate pharmaceutical industry by adding various substituents to the basic molecule in order to modify potency (Table 1). This approach has been mimicked by chemists in clandestine laboratories to produce illicit NPF derivatives. Depending on the position of the substituents, some of the resulting molecules may exist as enantiomers e.g. the isomers of 3-methylfentanyl, which have differing analgesic potencies depending on which enantiomer is used. Van Bever et al. (1974) reported that the introduction of a methyl group (-CH3) into the 3 position of the piperidine ring, increases analgesic potency. The trans isomer was slightly more active than fentanyl, but its corresponding cis form was eight times more active. They found that activity of the cis isomer resided in one enantiomer, namely cis(+) 3-methylfentanyl which was16 times more potent than fentanyl, whereas the cis(-) form was 120 times less potent (see non-pharmaceutical fentanyls below).
cis(+)3-methylfentanyl
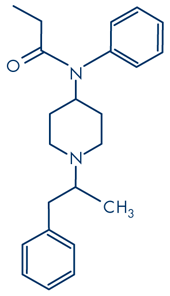
Alpha-methylfentanyl
trans (±)3-methylfentanyl
Physical form
Fentanyl and its salts are white granular or crystalline powders. Pharmaceutical formulations occur as solutions of fentanyl citrate for injection and in transdermal patches, as well as transmucosal lozenges. Illicit forms include a light yellow powder called ‘White Persian’ containing 3-methylfentanyl and occasionally ‘paper trips’ (thin pieces of cardboard impregnated with fentanyl).
Pharmacology
Fentanyl is a narcotic analgesic acting predominately at the µ-opiate receptor. Apart from analgesia, the fentanyls as a group produce drowsiness and euphoria, the latter being less pronounced than with heroin and morphine. The most common side effects include nausea, dizziness, vomiting, fatigue, headache, constipation, anaemia and peripheral oedema. Tolerance and dependence develop rapidly after repeated use. Characteristic withdrawal symptoms (sweating, anxiety, diarrhoea, bone pain, abdominal cramps, shivers or ‘goose flesh’) occur when use is stopped. Serious interactions can occur when fentanyls are mixed with heroin, cocaine, alcohol and other CNS depressants e.g. benzodiazepines. The use of HIV protease inhibitors such as Ritonavir has been reported to increase plasma levels and reduce elimination of co-administered fentanyl.
Overdose results in respiratory depression which is reversible with naloxone. Sudden death can also occur because of cardiac arrest or severe anaphylactic reaction. The estimated lethal dose of fentanyl in humans is 2 mg. The recommended serum concentration for analgesia is 1–2 ng/ml and for anaesthesia it is 10–20 ng/ml. Blood concentrations of approximately 7 ng/ml or greater have been associated with fatalities where poly-substance use was involved. While fatalities have been reported after therapeutic use, many deaths have occurred as a result of the misuse of pharmaceutical products. Both used and unused fentanyl patches have been injected, smoked, snorted or taken orally with fatal consequences.
Non-pharmaceutical fentanyls
A significant number of deaths have been reported in the EU and USA following the ingestion of illicitly synthesised or ‘designer’ fentanyls, sometimes referred to as non-pharmaceutical fentanyls (NPF). In the case of NPFs, many deaths — characterised by their suddenness — have been caused by the use of heroin laced with fentanyl or with one of its more potent analogues, such as alpha-methyl fentanyl and 3-methylfentanyl. Animal tests conducted by the Janssen Pharmaceutical research group showed that the analgesic potency of fentanyl was 470 times that of morphine, while alpha-methylfentanyl was 600 times more potent. However the cis(+) form of 3-methyl fentanyl was 6 684 times more potent and the trans(±) form approximately 500 times more potent than morphine in the same tests. Carfentanil is said to be 10 000 times more potent than morphine. It is difficult to be certain that this increased analgesic potency means that the euphoric effects are similarly increased, and more importantly, whether the overdose potential of these analogues is also increased by the same margin. Van Bever et al. (1974) stated that the cis(+) isomer of 3-methylfentanyl had a six times higher safety margin than fentanyl and a 22 times higher margin than morphine, based on animal studies. A more recent report by Higashikawa and Suzuki (2008) also based on animal tests, found that the range between the effective and lethal doses of α-methylfentanyl was narrower than that of fentanyl. They suggested that this could also contribute to the deaths from the former.
Using a method capable of measuring both of the 3-methylfentanyl isomers, a report on an epidemic of 3-methylfentanyl fatalities in Estonia (Ojanperä et al. (2008)) stated that the average combined concentration of the isomers was — at 1.9 ng/ml — approximately 10 times lower than the concentrations found in Canadian and US fatalities attributed solely to fentanyl (ranging from 17–25 ng/ml) (Hull et al. (2007), Martin et al. (2006).
|
Name |
Chemical name |
Chemical formula CAS Number |
Control status (1961 UN Convention) |
Medical use |
Pharmaceutical name |
|
Acetyl- |
N-[1-(α-methylphenethyl)- |
C22H28N2O 101860-00-8 |
Schedule I, IV |
None |
None |
|
Alfentanil |
N-[1-[2-(4-ethyl-4,5-dihydro- |
C21H32N6O3 69049-06-5 |
Schedule I |
Surgical analgesic/ anaesthetic |
Alfenta®, Rapifen® |
|
Alpha- |
N-[1-(α-methylphenethyl)- |
C23H30N2O 79704-88-4 |
Schedule I, IV |
None |
None |
|
Alpha- |
N-[1-[1-methyl-2-(2-thienyl)ethyl]- |
C21H28N2OS 103963-66-2 |
Schedule I, IV |
None |
None |
|
Beta- |
N-[1-(β- hydroxyphenethyl)- |
C22H28N2O2 78995-10-5 |
Schedule I, IV |
None |
None |
|
Beta-hydroxy-3 methylfentanyl |
N-[1-(β-hydroxyphenethyl)-3- |
C23H30N2O2 78995-14-9 |
Schedule I, IV |
None |
None |
|
Fentanyl |
1-phenethyl-4-N- |
C22H28N2O 437-38-7 |
Schedule I |
Analgesic, anaesthetic |
Sublimaze®, Actiq®, Durogesic®, Effentora® |
|
3-methylfentanyl |
N-(3-methyl-1-phenethyl- |
C23H30N2O 42045-86-3 |
Schedule I, IV |
None |
None |
|
3-methylthio- |
N-[3-methyl-1-[2-(2-thienyl)ethyl]- |
C21H28N2OS 86052-04-2 |
Schedule I, IV |
None |
None |
|
Para-fluoro- |
4′-fluoro-N-(1-phenethyl- |
C22H27FN2O 90736-23-5 |
Schedule I, IV |
None |
None |
|
Remifentanil |
1-(2-methoxycarbonylethyl)- |
C20H28N2O5 132539-07-2 |
Schedule I |
Short-acting analgesic during anaesthesia |
Ultiva® |
|
Sufentanil |
N-[4-(methoxymethyl)-1- |
C22H30N2OS 60561-17-3 |
Schedule I |
Analgesic in anaesthesia |
Sufenta® |
|
Thiofentanyl |
N-[1-[2-(2-thienyl)ethyl]- |
C20H26N2OS 60771-38-2 |
ScheduleI, IV |
None |
None |
|
Carfentanil |
4((1-oxopropyl)phenylamino)- |
C24H30N2O3 59708-52-0 |
Not Scheduled |
Immobilization of large animals (in veterinary practice) |
Wildnil® |
Synthesis
Janssen Pharmaceutical originally synthesised fentanyl from N-benzyl-4-piperidone but in the 1980s a new synthetic route became available based on 4-piperidone hydrochloride (CAS No 40064-34-4) by reacting it with phenethyl bromide to give N-phenethyl-4-piperidone (NPP) which is then converted to fentanyl. This is referred to as the Siegfried method of synthesis which uses either phenethyl-tosylate or phenethyl bromide to make the NPP precursor.
Mode of use
Intravenous injection (Sublimaze®), transdermal patches (Durogesic®), oral transmucosal lozenges (Actiq®), buccal tablets (Effentora ®). Non-prescribed fentanyl has been misused by injection; by oral ingestion of lozenges, patches (both used and unused) and ‘trips’ and fentanyl powder or patches have also been smoked or taken intranasally (snorted).
Other names
China White, Synthetic Heroin, Drop Dead, Flatline, Lethal Injection, Apache, China Girl, Chinatown, Dance Fever, Great Bear, Poison, Tango & Cash, TNT. Perc-o-Pops and Lollipops are street names for Actiq®.
Analysis
Fentanyl gives an orange colour in the Marquis field test. The mass spectrum shows a major ion at m/z =245 with other ions at m/z =146,42,189 and 44. Fentanyl in body fluids does not respond to immunoassay screening tests for morphine-type opioids. The limit of detection in plasma using gas chromatography is 20 ng/l.
Control status
Fentanyl has been controlled under Schedule I of the 1961 UN Single Convention on Narcotic Drugs since 1964. Other fentanyl derivatives which were added to Schedule I in 1980 include Sufentanil and Para-fluorofentanyl, while Alfentanil was added in 984 and Remifentanil in 1999. In all, 13 fentanyls are controlled under the 1961 Convention. In the US, the supply of NPP (CAS No 39742-60-4) has been controlled since July 2008 and the Drug Enforcement Administration has proposed controlling the anilino derivative of NPP (ANPP- CAS No 21409-26-7)) which is the immediate precursor of fentanyl. But 4-piperidone is not controlled either in the USA or within the EU. Furthermore, NPP and ANPP are not controlled in the EU Member States.
Availability of pharmaceutical fentanyls
World production of pharmaceutical fentanyl was 2.6 tonnes in 2005 according to the International Narcotics Control Board (INCB), with Belgium accounting for 47 % of production and the USA for 46 %. Smaller quantities were produced in South Africa, the UK and the Netherlands. Ireland was the largest importer (1 tonne) and the second largest exporter after Belgium. Alfentanil comes almost exclusively from Belgium which accounted for 91 % of the 25.2 kg produced in 2005. The UK is the leading producer of Remifentanil (92.6 kg globally in 2005), while the USA accounts for 81 % of the 3.6 kg of Sufentanil manufactured in 2005.
Publications
Infographics and media
Bibliography
Denton, J.S., Donoghue, E.R., McReynolds, J. and Kalelkar, M. (2008), 'An epidemic of illicit fentanyl deaths in Cook County Illinois: September 2005 through April 2007', Journal of Forensic Sciences, Volume 53, Issue 2, pp. 452–454.
Drug Enforcement Administration (DEA), US, Department of Justice (2007), 'Control of a chemical precursor used in the illicit manufacture of fentanyl as a List I chemical. Interim rule with request for comments', Federal Register, Volume 72, No 77, pp. 20039–47.
Henderson, G. (1991), 'Fentanyl-related deaths: demographics, circumstances and toxicology in 112 cases', Journal of Forensic Sciences, Volume 36, Issue 2, pp. 422–433.
Higashikawa, Y. and Suzuki, S. (2008), 'Studies on 1-(2-phenethyl)-4-(N-propionylanilino)piperidine (fentanyl) and its related compounds. VI. Structure-analgesic activity relationship for fentanyl, methyl-substituted fentanyls and other Analogues', Forensic Toxicology, Volume 26, No 1, pp. 1–5.
Hull, M.J., Juhascik, M., Mazur, F., Flomenbaum, M.A. and Behonick, G.S. (2007), 'Fatalities associated with fentanyl and co-administered cocaine or opiates', Journal of Forensic Sciences, Volume 52, Issue 6, pp. 1383–1388.
International Narcotics Control Board (2006), Psychotropic Substances: Statistics for 2004 – Assessments of Annual Medical and Scientific Requirements for Substances in Schedules II,III and IV, United Nations Publications, New York.
Jones, T.S., Krzywicki, L., Maginnis, J., et al. (contributors) (2008), 'Nonpharmaceutical fentanyl-related deaths – multiple States, April 2005–March 2007', Morbidity and Mortality Weekly Report, Volume 57, No 29, pp. 793–796, Centers for Disease Control and Prevention, USA. http://www.cdc.gov.mmwr/preview/mmwrhtml/mm5729a1.htm.
Kram, T.C., Cooper, D.A. and Allen, A.C. (1981), 'Behind the identification of China White', Analytical Chemistry, Volume 53, Issue 12, pp. 1379A–1386A, accessed through: www.erowid.org/archive/rhodium/chemistry/china.white.identification.html.
Kronstrand, R., Druid H., Holmgren, P. and Rajs, J. (1997), 'A cluster of fentanyl-related deaths among drug addicts in Sweden', Forensic Science International, Volume 88, No 3, pp. 185-195.
Moffat, A.C., Osselton, M.D. and Widdop, B. (editors) (2004), Clarke’s Analysis of Drugs and Poisons, 3rd edition, Volume 2, Pharmaceutical Press, London.
Ojanperä, I., Gergov, M., Liiv, M., Riikoja, A. and Vuori, E. (2008), 'An epidemic of fatal 3-methylfentanyl poisonings in Estonia', International Journal of Legal Medicine, E-publication DOI 10.1007/s00414-008-0230-x.
Ould-Ahmed, M., Drouillard, I., Savio, C., Baud, F.J., Migliani, R. and Michel, A. (1999), 'Intoxications aiguës prises en charge par un service mobile d’urgence et de reanimation: Description rétrospective de 361 cas' (in French and English), Réanimation Urgences, Volume 8, pp. 93–97.
Sweetman S.C. (editor) (2007), Martindale: the complete drug reference, 35th edition, Pharmaceutical Press, London.
United Nations (2006), Multilingual Dictionary of Narcotic Drugs and Psychotropic Substances under International Control, United Nations, New York.
Van Bever, W.F., Niemegeers, C.J. and Janssen, P.A. (1974), 'Synthetic Analgesics. Synthesis and pharmacology of the diastereoisomers of N-[3-Methyl-1-(2-phenylethyl)-4-piperidyl]-N-phenylpropanamide and N-[3-Methyl -1(1--methyl-2-phenethyl)-4-piperidyl]-N-phenylpropanamide, Journal of Medicinal Chemistry, Volume 17, pp. 1047–1051.
Von Mach, M-A., Kaes, J., Solak, E., Lauterbach, M. and Weilemann, L.S. (2006), 'Unerwünschte Arzneimittelwirkungen — Anfragen bei einem giftinformationszentrum von 1995 bis 2004', Deutsches Ärzteblatt, Volume 103, No 11, pp. A694–A700.
Woodall, K. L., Martin, T.L. and McLellan, B.A. (2008), 'Oral abuse of fentanyl patches (Duragesic®): seven case reports', Journal of Forensic Sciences, Volume 53, No 1, pp. 222–225.




















