Os canabinoides sintéticos na Europa
Introduction
Análise: os canabinoides sintéticos na Europa

Os agonistas dos recetores de canabinoides sintéticos (normalmente designados por «canabinoides sintéticos») são um grupo de substâncias que imitam os efeitos do (–)--Δ9-tetrahidrocanabinol (THC), a substância que é a principal responsável pela maioria dos efeitos psicoativos da canábis. À semelhança do THC, os canabinoides sintéticos ligam-se aos recetores de canabinoides no organismo. É por este motivo que estas substâncias são utilizadas para criar uma vasta gama de produtos «legal high» («drogas legais») vendidos como substitutos legais da canábis. Os canabinoides sintéticos constituem o maior grupo das novas substâncias psicoativas monitorizadas pelo Observatório Europeu da Droga e da Toxicodependência (EMCDDA).
Os chamados produtos «legal high» com canabinoides sintéticos são vendidos como «misturas de ervas para fumar» desde meados da década de 2000. Estes produtos não contêm canábis mas, quando fumados, produzem efeitos semelhantes. Estas substâncias têm sido objeto de abordagens de marketing inovadoras e estão amplamente disponíveis na Internet e, em alguns países, em lojas convencionais (frequentemente designadas por «head» ou «smart»).
O número de canabinoides sintéticos, a sua diversidade química e o ritmo a que surgem tornam este grupo de compostos particularmente difícil de detetar, monitorizar e dar resposta. Os fornecedores visam, simplesmente, imitar os efeitos do THC, o que, na prática, torna os canabinoides sintéticos descartáveis. Sempre que um canabinoide sintético é, ou está em vias de ser, controlado legalmente, os fabricantes podem ter uma ou várias substâncias substitutas prontas para serem vendidas.
Ainda pouco se sabe sobre a forma como estas substâncias funcionam e sobre os seus efeitos tóxicos para os seres humanos. No entanto, o seu consumo tem causado um elevado número de intoxicações graves e mesmo de mortes, algumas das quais resultantes de intoxicações em massa. É possível que, para além de serem muito potentes, algumas delas tenham meias-vidas longas e possam provocar efeitos psicoativos prolongados. Além disso, é provável que, pelo menos, algumas destas substâncias afetem as funções fisiológicas do organismo, para além dos efeitos sobre os recetores de canabinoides.
A presente análise visa fazer o ponto da situação acerca dos atuais conhecimentos sobre estas substâncias e os seus efeitos, bem como das tendências em matéria de produção, disponibilidade e consumo.
O aparecimento dos canabinoides sintéticos
Apesar dos rumores na Internet, desde meados da década de 2000, sobre a existência de «misturas de ervas para fumar» vendidas como «legal high» capazes de produzir «fortes» efeitos semelhantes aos da canábis, só em 2008 investigadores forenses detetaram pela primeira vez, na Alemanha e na Áustria, o canabinoide sintético JWH-018 num produto vendido sob a marca «Spice» (1). Subsequentemente, foram detetados vários canabinoides em misturas para fumar ou em pretensos incensos/ambientadores. Os produtos Spice Gold, Spice Silver e Yucatan Fire são exemplos típicos, mas depois deles apareceram muitos outros produtos. Muitos dos canabinoides detetados nesses produtos foram inicialmente desenvolvidos por cientistas que investigavam de que modo os canabinoides afetam o organismo e pretendiam determinar se estes poderiam ser utilizados no tratamento de doenças e dos respetivos sintomas, tais como doenças neurodegenerativas, toxicodependência, distúrbios dolorosos e cancro. Até agora, porém, tem sido difícil separar as propriedades terapêuticas desejadas dos efeitos psicoativos indesejados.
Os canabinoides sintéticos constituem o maior grupo de compostos atualmente monitorizados através do mecanismo de alerta rápido da UE. 1 em 2008, 9 em 2009, 11 em 2010, 23 em 2011, 30 em 2012, 29 em 2013, 30 em 2014, 25 em 2015 e 11 em 2016. No total, foram notificados ao EMCDDA, até dezembro de 2016 (2), 169 canabinoides sintéticos.
Os canabinoides sintéticos desempenham um papel importante no mercado em rápida mutação de «legal highs», um termo genérico utilizado para descrever (novas) substâncias psicoativas não regulamentadas que, geralmente, pretendem imitar os efeitos das drogas controladas e ser vendidas no mercado aberto. Existem poucos dados sobre o seu consumo, os riscos e danos que envolvem são em grande medida desconhecidos e as drogas muito potentes causam grande preocupação. As misturas para fumar que contêm canabinoides sintéticos, por exemplo, podem apresentar consideráveis variações de lote para lote ou dentro de um mesmo lote, quer em termos das substâncias presentes quer das suas concentrações.
O fabrico de produtos com canabinoides sintéticos
A maior parte dos canabinoides sintéticos utilizados nos produtos «legal highs» são fabricados por empresas químicas com sede na China. São expedidos sob a forma de pó a granel para a Europa, utilizando correio expresso e empresas privadas de correio; maiores quantidades podem ser expedidas por via aérea ou marítima. É frequente as autoridades europeias apreenderem remessas de vários quilos. Embora o grau de pureza destes pós a granel raramente seja determinado, um estudo da Coreia do Sul registou níveis de pureza de 75 % a 90 % nas amostras de pó a granel (3). Em 2015, mais de 24 000 apreensões de canabinoides sintéticos (24 210) foram feitas na Europa correspondendo a mais de 2,3 toneladas (2334 kg), das quais mais de 400 kg (444,245 kg) eram pó a granel. Isto representa um aumento de quase 7000 apreensões e mais de 1,6 toneladas (a maioria das quais consistindo em material vegetal) face a 2014.
Uma vez na Europa, são preparados os produtos para venda a retalho. A base vegetal a que são adicionadas as misturas para fumar é frequentemente constituída por damiana (Turnera diffusa) e plantas lamiaceae como a erva-cidreira, a menta e o tomilho (4). Os canabinoides sintéticos são misturados com o material vegetal ou polvilhados sobre este, normalmente numa escala industrial, utilizando solventes como a acetona ou o metanol para dissolver os pós; em seguida, são utilizados equipamentos como betoneiras para misturar os ingredientes. Depois, a mistura é seca e embalada para venda na Internet por retalhistas de produtos «legal high» e em lojas convencionais.
Devido à grande potência de alguns canabinoides sintéticos, a quantidade de pó necessária para cada pacote poderá não exceder algumas dezenas de miligramas. Por conseguinte, cada quilograma de pó a granel pode servir para produzir milhares de pacotes de produtos «legal high». A descoberta de instalações de transformação e embalagem, bem como de grandes quantidades de canabinoides sintéticos nos Países Baixos e na Bélgica, indica um envolvimento da criminalidade organizada no processo de distribuição. Também há indícios da existência, na Europa, de um significativo comércio retalhista baseado na Internet, sendo regularmente apreendidas pequenas quantidades destes produtos pelas autoridades aduaneiras e policiais.
A monitorização das lojas virtuais que vendem produtos «legal high» permite perceber melhor a variedade de misturas para fumar que nelas podem ser compradas, muitas das quais com canabinoides sintéticos. Essa monitorização, quando conjugada com a compra desses produtos à venda, para análise, permite igualmente acompanhar a evolução das substâncias presentes num produto ao longo do tempo e contribui para detetar precocemente os novos canabinoides que aparecem no mercado.
Prevalência
As informações disponíveis sobre a dimensão do consumo de produtos com canabinoides sintéticos são limitadas; no entanto, o conhecimento da situação está a melhorar, na medida em que mais países começaram a incluir perguntas sobre o consumo de novas drogas nos seus inquéritos à população em geral. Pelas informações atualmente disponíveis, afigura-se que a prevalência do seu consumo na população em geral é baixa na Europa. Foram lançados vários inquéritos destinados a examinar a prevalência do consumo de produtos de tipo «Spice», mas a sua cobertura e representatividade ainda são limitadas.
Há claras diferenças na prevalência do consumo de produtos com canabinoides sintéticos entre os mercados de droga europeus e americanos. Os mais recentes dados da prevalência nos EUA provêm do inquérito à população estudantil, realizado neste país, em 2014, e intitulado «Monitoring the Future», o qual sugere que o consumo está a diminuir, sendo a prevalência do consumo no último ano, entre os estudantes de 17/18 anos de idade, de 5,8 % em 2014, comparativamente a 7,9 % em 2013 e 11,3 % em 2012 (29). Em 2011, de acordo com o mesmo inquérito, a «marijuana sintética» era a segunda droga mais consumida a seguir à canábis, com uma prevalência no último ano de 11,4 %.
Vários inquéritos efetuados em países europeus também recolheram dados sobre o consumo de canabinoides sintéticos, mas esses dados não são comparáveis, já que utilizam métodos, bases de amostragem e terminologia diferentes. Em conjunto, esses estudos indicam níveis de prevalência muito baixos. O Reino Unido (Inglaterra e País de Gales) incluiu a pergunta sobre o consumo de «Spice» em dois inquéritos às famílias consecutivos e registou níveis de prevalência ao longo da vida entre a população adulta (16 a 64 anos) de 0,2 % em 2010/2011 e de 0,1 % em 2011/2012 (6,7). De acordo com o inquérito britânico sobre a criminalidade em Inglaterra e no País de Gales no período de 2014/2015, 0,9 % de adultos (16 a 59 anos) tinham consumido novas substâncias psicoativas no último ano, dos quais 61 % tinham consumido uma mistura de ervas para fumar (8). A pergunta não foi repetida nos anos seguintes devido à baixa taxa de prevalência.
Em Espanha, em 2014, um inquérito nacional sobre o consumo de drogas entre a população estudantil dos 14 aos 18 anos a uma amostra de 37 486 indivíduos identificou níveis reduzidos de consumo de produtos «Spice», com taxas de prevalência de 0,8 % em 2014 para consumo ao longo da vida, diminuindo de 1,4 % em 2012 e 1,1 % em 2010 (9,32). Num inquérito à população em geral realizado em 2013 também em Espanha, 0,5 % dos 23 136 inquiridos (de idades entre os 15 e os 64 anos) registou um consumo ao longo da vida de Spice (10).
Em França, um inquérito à população adulta (18 aos 64 anos) realizado em 2014, que incluía uma pergunta sobre o consumo de «canabinoides sintéticos», registou uma taxa de consumo ao longo da vida de 1,7 %. As pessoas que consomem estes novos produtos sintéticos pela primeira vez são, na sua maioria, homens (2,3 % contra 1,2 % de mulheres), e da geração mais jovem (menos de 35 anos): 4,0 % das pessoas com idades entre os 18 e os 34 anos experimentaram canabinoides sintéticos, em comparação com 0,6 % dos 35 aos 64 anos (11). Outro inquérito a jovens de 17 anos, realizado em França, concluiu que 1,7 % desses jovens já tinham consumido um canabinoide sintético (12).
O inquérito do Conselho Sueco de Informação sobre Álcool e outras Drogas (CAN) de 2015 inquiria a população estudantil sobre o consumo de novas substâncias psicoativas. Registou uma diminuição comparativamente com outros anos, nos 9.º e 11.º anos, com 1,6 % e 3,2 %, respetivamente, a registarem consumo em determinada altura. Os canabinoides sintéticos eram as novas substâncias psicoativas mais utilizadas por jovens no 9.º ano e no segundo ano do nível secundário superior (37).
Na Alemanha, a câmara municipal de Francoforte estudou a utilização de misturas para fumar e de «Spice» entre a população estudantil dos 15 aos 18 anos, tendo registado níveis de consumo ao longo da vida de 7 % em 2009, 9 % em 2010 e 7 % em 2011 e 2012 (13,14,15). Em 2013, o consumo ao longo da vida de misturas para fumar reduziu para 5 %, mas aumentou para 6 % em 2014 e manteve-se nos 6 % em 2015. Contudo, continua abaixo dos valores registados entre 2009 e 2012 (16,17, 33). Os estudantes que mencionaram o consumo de «Spice» eram, na sua maioria, consumidores experimentados de canábis.
Por último, vários estudos levados a cabo em grupos específicos (frequentadores de clubes noturnos, utilizadores da Internet, etc.), utilizando amostras não-probabilísticas, identificaram níveis mais elevados de consumo de canabinoides sintéticos do que entre a população em geral. O Global Drug Survey de 2012, por exemplo, registou níveis de prevalência no último ano de 3,3 % entre todas as pessoas que responderam ao inquérito no Reino Unido (não representativas da população em geral) e de 5,0 % entre as que frequentam regularmente os clubes noturnos desse país (18).
No Reino Unido, o consumo de canabinoides sintéticos entre reclusos é particularmente preocupante. Um inquérito realizado em 2016 nos estabelecimentos prisionais do Reino Unido concluiu que 33 % dos 625 reclusos comunicaram o consumo de «Spice» no mês anterior (comparativamente com 14 % do consumo de canábis no mês anterior). Os níveis de prevalência do consumo de «Spice» no mês anterior entre estabelecimentos prisionais variava entre os 15 % e os 71 %. Aos indivíduos que tinham consumido «Spice» no mês anterior foi perguntado sobre a frequência semanal de consumo. Os resultados indicaram que 31 % tinham consumido «Spice» uma ou duas vezes por semana, 8 % uma vez por semana, 15 % 2 a 3 vezes por semana e 46 % quase diariamente (30). Um estudo anterior realizado em 2015 pelo serviço de inspeção prisional do Reino Unido (HM Inspectorate of Prisons) em que participaram 1 376 reclusos em oito estabelecimentos concluiu que 10 % consumiam «Spice» nos respetivos estabelecimentos (31).
Consequências adversas para a saúde associadas aos canabinoides sintéticos
Os efeitos adversos para a saúde que são associados aos canabinoides sintéticos prendem-se com as propriedades intrínsecas dessas substâncias, com o que o organismo faz às substâncias e com a forma como os produtos são fabricados. Já se registaram muitos casos de intoxicações não fatais e um número mais pequeno de mortes associadas ao seu consumo (19,20). Tendo em conta a elevada potência de alguns destes compostos, a probabilidade de que produzam efeitos tóxicos parece ser elevada. Estes riscos podem ser agravados pelo processo de fabrico, do qual poderá resultar uma distribuição desigual das substâncias presentes no material vegetal. Tal poderá fazer com que alguns produtos contenham partes («hot pockets») com concentrações elevadas de canabinoides, dando origem a doses superiores ao previsto e aumentando o risco de ocorrências adversas com efeitos graves (21,22). É igualmente provável que alguns desses efeitos adversos se devam a outros mecanismos que não a interação com os recetores de canabinoides, como, por exemplo, à interferência com outras funções fisiológicas no organismo (23).
Um estudo sistemático recente das ocorrências adversas associadas aos produtos com canabinoides sintéticos concluiu que os sintomas de intoxicação mais referidos eram a agitação, a náusea e um ritmo cardíaco anormalmente acelerado (17), enquanto outras ocorrências adversas com efeitos graves (tais como derrames, convulsões, ataques cardíacos, falência dos tecidos musculares, lesões nos rins, psicoses e vómito grave ou prolongado) e as mortes associadas eram menos comuns (17). Foram também referidos sintomas que sugerem dependência e abstinência (22). De um modo geral, é difícil calcular até que ponto estes efeitos adversos são comuns porque, entre outros fatores, se desconhece o número total de pessoas expostas às drogas (17).
Uma das características mais marcantes dos produtos com canabinoides sintéticos é a sua capacidade para provocar surtos de intoxicações em massa. Por vezes, são afetadas centenas de pessoas durante um curto período de tempo, um problema grave que se manifestou nos últimos anos nos Estados Unidos e na Rússia. Na Rússia, em 2014, o canabinoide MDMB-FUBINACA foi associado a mais de 600 intoxicações, incluindo 15 mortes, durante um período de duas semanas (23). No início de 2016, esta substância foi identificada no mercado europeu, motivando um alerta do EMCDDA em matéria de saúde pública para a sua rede de alerta rápido. Em 2015, ocorreu outro grande surto nos Estados Unidos, que parece ter estado parcialmente associado a uma substância denominada ADB-FUBINACA (24,25). Embora estes tipos de surtos aparentem ser raros na Europa, em 2015, na Polónia, foram comunicadas mais de 200 situações de urgência hospitalar em menos de uma semana depois de as pessoas terem afirmado que tinham fumado um produto denominado «Mocarz».
Em julho de 2016, o MDMB-CHMICA foi o primeiro agonista dos recetores de canabinoides sintéticos a ser sujeito a uma avaliação dos riscos pelo EMCDDA (35) e recentemente foi sujeito a medidas de controlo e sanções penais em toda a Europa (36). O MDMB-CHMICA está classificado como um potente agonista integral do recetor CB1 e demonstrou ser também um agonista do recetor CB2. Na altura em que foi efetuada a avaliação de riscos, foram comunicadas ao EMCDDA 25 intoxicações agudas e 28 mortes associadas ao MDMB-CHMICA. O MDMB-CHMICA foi responsável ou terá contribuído para 12 dos casos de morte. Em três casos, foi mesmo a única substância detetada. O MDMB-CHMICA está disponível no mercado de drogas europeu desde, pelo menos, agosto de 2014, e na altura em que a avaliação de riscos foi efetuada, tinha sido detetado em 23 Estados-Membros, na Turquia e Noruega. Informações fornecidas ao EMCDDA e à Europol registavam a apreensão de mais de 120 kg de MDMB-CHMICA com aproximadamente 67 kg e 46 kg sob a forma de material vegetal e de pó, respetivamente. A maior apreensão única de produto a granel notificada ao EMCDDA, foi de 40 kg de pó de pureza extremamente elevada contendo MDMB-CHMICA, proveniente da China (34).
A monitorização de ocorrências adversas com efeitos graves pelo EMCDDA e os conhecimentos atuais dos efeitos farmacológicos e toxicológicos de alguns canabinoides sintéticos mostram que esses compostos podem prejudicar gravemente a saúde humana, embora os mecanismos envolvidos ainda sejam insuficientemente compreendidos.
Evolução recente
Desde o início do fenómeno dos canabinoides sintéticos, estas substâncias têm sido detetadas em grande número em produtos vendidos como «misturas de ervas para fumar». Mais recentemente, porém, vários países também comunicaram a sua deteção em produtos semelhantes a resina de canábis vendidos como «legal high», caso do «Incenso Afegão», ou no mercado de drogas ilícitas, como meras imitações da resina de canábis. Esta evolução terá provavelmente a ver com a popularidade desta droga em muitos países. Também foram detetados canabinoides sintéticos em misturas que continham outras substâncias psicoativas novas, designadamente estimulantes, alucinogénios e sedativos/hipnóticos; esta situação tanto pode ser deliberada como acidental. Num pequeno número de casos, a presença de canabinoides sintéticos foi detetada em comprimidos ou cápsulas que pareciam ser de ecstasy. Na Hungria e nos Estados Unidos, estes deram origem a grupos de intoxicações graves (26). Outra evolução recente foi a descoberta de canabinoides sintéticos nas recargas de líquido utilizadas em cigarros eletrónicos, a qual reflete, muito provavelmente, a popularidade ultimamente adquirida pelo «vaping» entre os jovens.
O mecanismo de alerta rápido do EMCDDA tem vindo a monitorizar atentamente a evolução dos canabinoides sintéticos desde a sua identificação no mercado europeu em 2008. Uma característica distintiva desta família química é o modo como tem evoluído e se tem adaptado ao longo deste período. Torna-se evidente que os inovadores padrões de substituição química que têm caracterizado este fenómeno exigirão uma monitorização apertada e contínua da evolução no terreno, nomeadamente dos danos causados pelos canabinoides sintéticos.
Footnotes
- (1) EMCDDA (2009), Understanding the ‘Spice’ phenomenon, Documento temático do EMCDDA, Serviço das Publicações da União Europeia, Luxemburgo.
- (2) Para efeitos de monitorização no âmbito do mecanismo de alerta rápido da UE, a expressão «canabinoides sintéticos» utilizada no presente documento inclui: o elevado número de substâncias agonistas dos recetores de canabinoides sintéticos (por exemplo, a substância JWH-018, que é um agonista dos recetores CB1 e CB2) detetado no mercado de drogas europeu; um número muito menor de moduladores alostéricos (por exemplo, Org 27569) que alteram a estrutura dos recetores de canabinoides e originam uma atividade alterada quando um ligante se liga aos recetores; e substâncias que atuam como inibidores da hidrólase das amidas de ácidos gordos (FAAH), que é a enzima responsável pela destruição do endocanabinoide anandamida (por exemplo, URB597). Este número das «Perspetiva sobre drogas» aborda apenas os agonistas de recetores de canabinoides sintéticos.
- (3) Choi, H., Heo, S., Choe, S., et al. (2013), «Simultaneous analysis of synthetic cannabinoids in the materials seized during drug trafficking using GC-MS», Analytical and Bioanalytical Chemistry, 405(12), pp. 3937–3944.
- (4) Ogata, J., Uchiyama, N., Kikura-Hanajiri, R. e Goda, Y. (2013), «DNA sequence analyses of blended herbal products including synthetic cannabinoids as designer drugs», Forensic Science International, 227(1–3), pp. 33–41.
- (5) National Institute on Drug Abuse (2014), «Monitoring the Future Survey 2014, overview of findings», NIDA, Bethesda, MD.
- (6) Smith, K. e Flatley, J. (eds) (2011), Drug misuse declared: Findings from the 2010/11 British Crime Survey, England and Wales, Home Office, Londres.
- (7) Office for National Statistics (2012), Drug misuse declared: Findings from the 2011/12 Crime Survey for England and Wales, Home Office, Londres.
- (8) Home Office (2015), «Tables for drug misuse: Findings from the 2014 to 2015 CSEW», Home Office, Londres.
- (9) Observatorio Español sobre Drogas (2012), Encuesta Estatal sobre Uso de Drogas en Enseñanzas Secundarias (ESTUDES) 2012.
- (10) Observatorio Español sobre Drogas (2013), Encuesta Domicilaria sobre Alcohol y Drogas en España (EDADES)
- (11) Beck, F., Richard, J.-B., Guignard, R., Le Nezet, O. e Spilka, S. (2015), «Les niveaux d'usage des drogues en France en 2014», Tendances 99.
- (12) Spilka, S., Le Nézet, O., Ngantcha, M. e Beck, F. (2015), «Les drogues à 17 ans : analyse de l’enquête ESCAPAD», Tendances 100.
- (13) Werse, B., Müller, O., Schell, C. e Morgenstern, C. (2011), Jahresbericht MoSyD: Drogentrends in Frankfurt am Main 2010, Centre for Drug Research, Frankfurt am Main.
- (14) Werse, B., Bernard, C., Schell-Mack, C. e Morgenstern, C. (2012), MoSyD Jahresbericht 2011: Drogentrends in Frankfurt am Main, Centre for Drug Research, Frankfurt am Main.
- (15) Bernard, C., Werse, B. e Schell-Mack, C. (2013), MoSyD Jahresbericht 2012: Drogentrends in Frankfurt am Main, Centre for Drug Research, Frankfurt am Main.
- (16) Werse, B., Morgenstern, C. e Sarvari, L. (2014), MoSyD Jahresbericht 2013: Drogentrends in Frankfurt am Main, Centre for Drug Research, Frankfurt am Main.
- (17) Werse, B., Kamphausen, G., Egger, D., Sarvari, L. e Müller, D. (2015), MoSyD Jahresbericht 2014: Drogentrends in Frankfurt am Main, Centre for Drug Research, Frankfurt am Main.
- (18) Guardian/Mixmag Survey (2012), acedido em 13 de março de 2013.
- (19) Tait, R. J., Caldicott, D., Mountain, D., Hill, S. L., Lenton, S. (2016), «A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment», Clinical Toxicology (Philadelphia) 54(1), pp. 1–13.
- (20) American Association of Poison Control Centers (n.d.), «Synthetic cannabinoids», AAPCC, Alexandria, VA.
- (21) Lindigkeit, R. 1., Boehme, A., Eiserloh, I., et al. (2009), «Spice: A never ending story?», Forensic Science International, 191(1–3), pp. 58–63.
- (22) Uchiyama, N., Kikura-Hanajiri, R., Ogata, J. e Goda, Y. (2010), «Chemical analysis of synthetic cannabinoids as designer drugs in herbal products», Forensic Science International, 198(1–3), pp. 31–38.
- (23) Fisar, Z. (2010), «Inhibition of monoamine oxidase activity by cannabinoids», Naunyn Schmiedeberg’s Archives of Pharmacology, 381(6), pp. 563–572.
- (24) Macfarlane, V. e Christie, G. (2015), «Synthetic cannabinoid withdrawal: A new demand on detoxification services», Drug and Alcohol Review 34(2), pp. 147–153.
- (25) Shevyrin, V., Melkozerov, V., Nevero, A., et al. (2016), «Identification and analytical characteristics of synthetic cannabinoids with an indazole-3-carboxamide structure bearing a N-1-methoxycarbonylalkyl group», Analytical and Bioanalytical Chemistry 407(21), pp. 6301–6315.
- (26) Kasper, A. M., Ridpath, A. D., Arnold, J. K., et al. (2015), «Severe illness associated with reported use of synthetic cannabinoids: Mississippi, April 2015», Morbidity and Mortality Weekly Report 64(39), pp. 1121–1122.
- (27) Drug Enforcement Administration (2015), «Proposed rule schedules of controlled substances: Temporary placement of the synthetic cannabinoid MAB-CHMINACA into Schedule I», Federal Register 80(179), pp. 55565–55568.
- (28) Brenneman, R., Papsun, D. M., Logan, B. K. e Neavyn, M. J. (2016), «Death-like slumber: Toxic outbreak of AB-FUBINACA», Journal of Medical Toxicology, 12(1), p. 39.
- (29) Johnston, L. D., O'Malley, P. M., Miech, R. A., Bachman, J. G., & Schulenberg, J. E. (2016). Monitoring the Future national survey results on drug use, 1975-2015: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan, pp. 98. Disponível em: http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf.
- (30) User Voice (2016), Spice: the bird killer. What prisoners think about the use of spice and other legal highs in prison. Disponível em: http://www.uservoice.org/news/user-voice-news-blog/2016/05/nhs-report-by-user-voice-hears-directly-from-inmates-the-true-horrors-of-nps-use-in-prisons/.
- (31) HM Inspectorate of Prisons (2015). Changing patterns of substance misuse in adult prisons and service responses. A thematic review by HM Inspectorate of Prisons, dezembro de 2015. Disponível em: https://www.justiceinspectorates.gov.uk/hmiprisons/wp-content/uploads/sites/4/2015/12/Substance-misuse-web-2015.pdf.
- (32) Observatorio Español sobre Drogas (2016) ESTUDES 2014/15. USID Encuesta sobre uso de drogas en enseñanzas secundarias en España. Disponível em: http://www.pnsd.msssi.gob.es/profesionales/sistemasInformacion/sistemaInformacion/pdf/2016_Informe_ESTUDES.pdf.
- (33) Werse, B., Egger, D., Sarvari, L., Kamphausen, G., e Müller, D. (2016), MoSyD Jahresbericht 2015: Drogentrends in Frankfurt am Main, Centre for Drug Research, Frankfurt am Main.
- (34) EMCDDA–Europol Joint Report on methyl. 2-[[1-(cyclohexylmethyl)indole-3-carbonyl]amino]-3,3-dimethylbutanoate (MDMB-CHMICA). EMCDDA–Europol , Lisboa, julho de 2016. Disponível em: http://emcdda.europa.eu/publications/joint-reports/mdmb-chmica.
- (35) EMCDDA (2017). Report on the risk assessment of methyl 2-[[1-(cyclohexylmethyl)-1H-indole-3-carbonyl]amino]-3,3-dimethylbutanoate in the framework of the Council Decision on new psychoactive substances. Disponível em: [inserir link sempre que disponível].
- (36) Decisão do Conselho (UE) 2017/369 de 27 de fevereiro 2017 sobre a submissão da metil 2-[[1-(ciclo-hexilmetil)-1H-indol-3-carbonil]amino]-3,3-dimetilbutanoato (MDMB-CHMICA) a medidas de controlo. Jornal Oficial da União Europeia L 56/210, 3.3.2017, disponível em: http://eur-lex.europa.eu/legal-content/pt/TXT/HTML/?uri=CELEX:32017D0369&qid=1489767473947&from=pt.
- (37) Agência de Saúde Pública da Suécia (2016), Drugs workbook, Estocolmo, não publicado.
Interativo: desmistificação da química
A carregar o elemento interativo… aguarde
Para tornar a química dos canabinoides sintéticos mais fácil de compreender, apresenta-se aqui um modelo para ajudar a explicar a constituição química destes compostos. Os canabinoides sintéticos são quimicamente diferentes; o que têm em comum é a capacidade de se ligarem aos recetores de canabinoides. Contudo, a estrutura da maioria dos canabinoides sintéticos pode ser dividida em quatro grandes partes: o núcleo e substituintes, a ligação, o anel e substituintes, e a cauda. Selecionando a combinação correta de moléculas constituintes, surgirá um canabinoide sintético.
Sugestão: volte a clicar numa molécula para «alternar» o estado da seleção.
| Cannabinoid | Core | Core Substituent | Link | Ring system | Ring substituent | Tail |
|---|---|---|---|---|---|---|
| Apinaca | indazole | : | carboxamide | adamantyl | : | pentyl |
| 5F-APINACA (5F-AKB48) | indazole | : | carboxamide | adamantyl | : | 5-fluoropentyl |
| AM-2201 indazolecarboxamide analogue | indazole | : | carboxamide | naphthyl | : | 5-fluoropentyl |
| A-834,735 | indole | : | methanone | cyclopropyl | 2,2,3,3-tetramethyl | tetrahydropyran-4-yl methyl |
| JWH-015 | indole | : | methanone | naphthyl | : | propyl |
| AM-679 | indole | : | methanone | phenyl | 2-iodo | pentyl |
| Apica | indole | : | carboxamide | adamantyl | : | pentyl |
| JWH-018 | indole | : | methanone | naphthyl | : | pentyl |
| JWH-007 | indole | 2-methyl (core-substituent) | methanone | naphthyl | : | pentyl |
| JWH-018 adamantoyl derivative | indole | : | methanone | adamantyl | : | pentyl |
| AM-6527 | indole | : | carboxamide | naphthyl | : | pentyl |
| AM-6527 5F derivative | indole | : | carboxamide | naphthyl | : | 5-fluoropentyl |
| PB-22 | indole | : | carboxylate | quinolinyl | : | pentyl |
| JWH-081 | indole | : | methanone | naphthyl | 4-methoxy | pentyl |
| JWH-122 | indole | : | methanone | naphthyl | 4-methyl | pentyl |
| JWH-182 | indole | : | methanone | naphthyl | 4-propyl | pentyl |
| JWH-203 | indole | : | ethanone | phenyl | 2-chloro | pentyl |
| JWH-210 | indole | : | methanone | naphthyl | 4-ethyl | pentyl |
| JWH-250 | indole | : | ethanone | phenyl | 2-methoxy | pentyl |
| JWH-251 | indole | : | ethanone | phenyl | 2-methyl (ring-substituent) | pentyl |
| JWH-387 | indole | : | methanone | naphthyl | 4-bromo | pentyl |
| JWH-398 | indole | : | methanone | naphthyl | 2-chloro | pentyl |
| JWH-412 | indole | : | methanone | naphthyl | 4-fluoro | pentyl |
| RCS-4 | indole | : | methanone | phenyl | 4-methoxy | pentyl |
| RCS-4 ortho isomer | indole | : | methanone | phenyl | 2-methoxy | pentyl |
| UR-144 | indole | : | methanone | cyclopropyl | 2,2,3,3-tetramethyl | pentyl |
| JWH-022 | indole | : | methanone | naphthyl | : | pent-4-enyl |
| JWH-122 pentenyl 2-methylindole derivative | indole | 2-methyl (core-substituent) | methanone | naphthyl | 4-methyl | pent-4-enyl |
| JWH-122 pentenyl derivative | indole | : | methanone | naphthyl | 4-methyl | pent-4-enyl |
| UR -144 (-2H) | indole | : | methanone | cyclopropyl | 2,2,3,3-tetramethyl | pent-4-enyl |
| AM-1220 Azepane Isomer | indole | : | methanone | naphthyl | : | methylazepan-3-yl |
| AB-005 azepane isomer | indole | : | methanone | cyclopropyl | 2,2,3,3-tetramethyl | methylazepan-3-yl |
| 3-(p-Methoxybenzoyl)-N-methylindole | indole | : | methanone | phenyl | 4-methoxy | methyl |
| JWH-019 | indole | : | methanone | naphthyl | : | hexyl |
| UR-144 heptyl derivative | indole | : | methanone | cyclopropyl | 2,2,3,3-tetramethyl | heptyl |
| BB-22 | indole | : | carboxylate | quinolinyl | : | cyclohexylmethyl |
| JWH-073 | indole | : | methanone | naphthyl | : | butyl |
| JWH-073 methyl derivative | indole | : | methanone | naphthyl | 4-methyl | butyl |
| RCS-4(C4) | indole | : | methanone | phenyl | 4-methoxy | butyl |
| 5FUR-144 | indole | : | methanone | cyclopropyl | 2,2,3,3-tetramethyl | 5-fluoropentyl |
| AM-2201 | indole | : | methanone | naphthyl | : | 5-fluoropentyl |
| AM-694 | indole | : | methanone | phenyl | 2-iodo | 5-fluoropentyl |
| AM-694 ethyl substituted for iodine | indole | : | methanone | phenyl | 2-ethyl | 5-fluoropentyl |
| AM-694 methyl substituted for iodine | indole | : | methanone | phenyl | 2-methyl (ring-substituent) | 5-fluoropentyl |
| MAM-2201 | indole | : | methanone | naphthyl | 4-methyl | 5-fluoropentyl |
| STS-135 | indole | : | carboxamide | adamantyl | : | 5-fluoropentyl |
| EAM-2201 | indole | : | methanone | naphthyl | 4-ethyl | 5-fluoropentyl |
| 5F-PB22 | indole | : | carboxylate | quinolinyl | : | 5-fluoropentyl |
| AM-694 chloro derivative | indole | : | methanone | phenyl | 2-iodo | 5-chloropentyl |
| JWH 018 N-(5-chloropentyl) derivative | indole | : | methanone | naphthyl | : | 5-chloropentyl |
| MAM-2201 chloropentyl derivative | indole | : | methanone | naphthyl | 4-methyl | 5-chloropentyl |
| UR-144 N-(5-chloropentyl) derivative | indole | : | methanone | cyclopropyl | 2,2,3,3-tetramethyl | 5-chloropentyl |
| JWH 018 N-(5-bromopentyl) derivative | indole | : | methanone | naphthyl | : | 5-bromopentyl |
| AM-2232 | indole | : | methanone | naphthyl | : | 4-cyanobutyl |
| A-796,260 | indole | : | methanone | cyclopropyl | 2,2,3,3-tetramethyl | 2-morpholin-4-yl ethyl |
| JWH-200 | indole | : | methanone | naphthyl | : | 2-morpholin-4-yl ethyl |
| WIN 48,098 / Pravadoline | indole | 2-methyl (core-substituent) | methanone | phenyl | 4-methoxy | 2-morpholin-4-yl ethyl |
| AB-005 | indole | : | methanone | cyclopropyl | 2,2,3,3-tetramethyl | methylpiperidin-2-yl methyl |
| AM-1220 | indole | : | methanone | naphthyl | : | methylpiperidin-2-yl methyl |
| AM-1248 | indole | : | methanone | adamantyl | : | methylpiperidin-2-yl methyl |
| AM-1248 azepane isomer | indole | : | methanone | adamantyl | : | methylazepan-3-yl |
| AM-2233 | indole | : | methanone | phenyl | 2-iodo | methylpiperidin-2-yl methyl |
| JWH-250 1-(2-methylene-N-methyl-piperidyl) derivative | indole | : | ethanone | phenyl | 2-methoxy | methylpiperidin-2-yl methyl |
| CRA-13 | naphthalene | : | methanone | naphthyl | : | pentoxy |
| JWH-307 | pyrrole | 5-(2-fluoro)phenyl | methanone | naphthyl | : | pentyl |
| JWH-370 | pyrrole | 5-(2-methyl)phenyl | methanone | naphthyl | : | pentyl |
| JWH-368 | pyrrole | 5-(3-fluoro)phenyl | methanone | naphthyl | : | pentyl |
| JWH-307 bromine analogue | pyrrole | 5-(2-bromo)phenyl | methanone | naphthyl | : | pentyl |
| JWH-030 | pyrrole | : | methanone | naphthyl | : | pentyl |
| JWH-145 | pyrrole | 5-phenyl | methanone | naphthyl | : | pentyl |
| AB-PINACA | indazole | : | carboxamide | carbamoyl | isopropyl | pentyl |
| ADB-FUBINACA | indazole | : | carboxamide | carbamoyl | tert-butyl | fluorobenzyl |
| ADB-PINACA | indazole | : | carboxamide | carbamoyl | tert-butyl | pentyl |
| AB-CHMINACA | indazole | : | carboxamide | carbamoyl | isopropyl | cyclohexylmethyl |
| ADB-CHMINACA | indazole | : | carboxamide | carbamoyl | tert-butyl | cyclohexylmethyl |
| MDMB-CHMICA | indole | : | carboxamide | methoxycarbonyl | tert-butyl | cyclohexylmethyl |
| 5F-MDMB-PINACA | indazole | : | carboxamide | methoxycarbonyl | tert-butyl | 5-fluoropentyl |
| MDMB-FUBINACA | indazole | : | carboxamide | methyl-3,3-dimethylbutanoate | : | fluorobenzyl |
| CUMYL-4CN-BINACA | indazole | : | carboxamide | CUMYL | : | 4-cyanobutyl |
| CUMYL-4CN-BINACA | indazole | : | carboxamide | CUMYL | : | 4-cyanobutyl |
| MO-CHMINACA | indazole | : | carboxylate | methoxycarbonyl | tert-butyl | cyclohexylmethyl |
| Apinaca | cannabinoid |
/sites/default/files/Apinaca.png |
371 | 517 | apinaca |
| 5F-APINACA (5F-AKB48) | cannabinoid | /sites/default/files/AKB-48Fv2.png | 391 | 528 | akb48f |
| AM-2201 indazolecarboxamide analogue | cannabinoid | /sites/default/files/AM-2201%2520indazolecarboxamide.png | 425 | 528 | am2201inda |
| A-834,735 | cannabinoid | /sites/default/files/A-834_735.png | 357 | 396 | a834735 |
| JWH-015 | cannabinoid | /sites/default/files/JWH-015.png | 356 | 455 | jwh015 |
| AM-679 | cannabinoid | /sites/default/files/AM-679.png | 356 | 425 | am679 |
| Apica | cannabinoid | /sites/default/files/apicaV2.png | 371 | 517 | apica |
| JWH-018 | cannabinoid | /sites/default/files/jwh-018v2.png | 356 | 517 | jwh018 |
| JWH-007 | cannabinoid | /sites/default/files/JWH-007.png | 356 | 517 | jwh007 |
| JWH-018 adamantoyl derivative | cannabinoid | /sites/default/files/JWH-018-adamantoyl-derivativeV2.png | 356 | 467 | jwh018adade |
| AM-6527 | cannabinoid | /sites/default/files/AM-6527.png | 425 | 517 | am6527 |
| PB-22 | cannabinoid | /sites/default/files/PB-22.png | 425 | 517 | pb22 |
| JWH-081 | cannabinoid | /sites/default/files/JWH-081.png | 445 | 517 | jwh081 |
| JWH-122 | cannabinoid | /sites/default/files/JWH-122.png | 409 | 517 | jwh122 |
| JWH-182 | cannabinoid | /sites/default/files/JWH-182.png | 498 | 517 | jwh182 |
| JWH-203 | cannabinoid | /sites/default/files/JWH-203.png | 356 | 517 | jwh203 |
| JWH-210 | cannabinoid | /sites/default/files/JWH-210.png | 445 | 517 | jwh210 |
| JWH-250 | cannabinoid | /sites/default/files/JWH-250.png | 409 | 517 | jwh250 |
| JWH-251 | cannabinoid | /sites/default/files/JWH-251.png | 356 | 517 | jwh251 |
| JWH-387 | cannabinoid | /sites/default/files/JWH-387.png | 409 | 517 | jwh387 |
| JWH-398 | cannabinoid | /sites/default/files/JWH-398.png | 356 | 517 | jwh398 |
| JWH-412 | cannabinoid | /sites/default/files/JWH-412.png | 409 | 517 | jwh412 |
| RCS-4 | cannabinoid | /sites/default/files/RCS-4.png | 445 | 425 | rcs4 |
| RCS-4 ortho isomer | cannabinoid | /sites/default/files/RCS-4%2520ortho%2520isomer.png | 356 | 425 | rcs4oriso |
| UR-144 | cannabinoid | /sites/default/files/UR-144.png | 350 | 447 | ur144 |
| JWH-022 | cannabinoid | /sites/default/files/JWH-022.png | 356 | 517 | jwh022 |
| JWH-122 pentenyl 2-methylindole derivative | cannabinoid | /sites/default/files/JWH-122%2520pentenyl%25202-methylindole.png | 409 | 517 | jwh122p2md |
| JWH-122 pentenyl derivative | cannabinoid | /sites/default/files/JWH-122%2520pentenyl.png | 409 | 517 | jwh122pd |
| UR -144 (-2H) | cannabinoid | /sites/default/files/UR-144%2520(-2H).png | 350 | 447 | ur1442h |
| AM-1220 Azepane Isomer | cannabinoid | /sites/default/files/AM-1220%2520azepane%2520isomer.png | 356 | 513 | am1220azis |
| AB-005 azepane isomer | cannabinoid | /sites/default/files/AB-005%2520azepane%2520isomer.png | 350 | 443 | ab005azeiso |
| 3-(p-Methoxybenzoyl)-N-methylindole | cannabinoid | /sites/default/files/3-%28p-methoxybenzoyl%29-N-methylindole.png | 445 | 301 | 3pmnm |
| JWH-019 | cannabinoid | /sites/default/files/JWH-019.png | 391 | 528 | jwh019 |
| UR-144 heptyl derivative | cannabinoid | /sites/default/files/UR-144%2520heptyl.png | 408 | 509 | ur144hep |
| BB-22 | cannabinoid | /sites/default/files/BB-22.png | 425 | 466 | bb22 |
| JWH-073 | cannabinoid | /sites/default/files/JWH-073.png | 356 | 466 | jwh073 |
| JWH-073 methyl derivative | cannabinoid | /sites/default/files/JWH-073%2520methyl.png | 409 | 466 | jwh073met |
| RCS-4(C4) | cannabinoid | /sites/default/files/RCS-4%2520(C4)(1).png | 445 | 374 | rcs4c4 |
| 5FUR-144 | cannabinoid | /sites/default/files/5FUR-144.png | 391 | 458 | 5fur144 |
| AM-2201 | cannabinoid | /sites/default/files/AM-2201.png | 391 | 528 | am2201 |
| AM-694 | cannabinoid | /sites/default/files/AM-694.png | 391 | 436 | am694 |
| AM-694 ethyl substituted for iodine | cannabinoid | /sites/default/files/AM-694%2520ethyl%2520for%2520iodine.png | 391 | 436 | am694ethio |
| AM-694 methyl substituted for iodine | cannabinoid | /sites/default/files/AM-694%2520methyl%2520for%2520iodine.png | 391 | 436 | am694methio |
| MAM-2201 | cannabinoid | /sites/default/files/MAM-2201.png | 409 | 528 | mam2201 |
| STS-135 | cannabinoid | /sites/default/files/STS-135v2.png | 391 | 528 | sts135 |
| EAM-2201 | cannabinoid | /sites/default/files/EAM-2201.png | 445 | 528 | eam2201 |
| 5F-PB22 | cannabinoid | /sites/default/files/5F-PB-22.png | 425 | 528 | 5fpb22 |
| AM-694 chloro derivative | cannabinoid | /sites/default/files/AM-694%2520chloro.png | 391 | 436 | am694chlo |
| JWH 018 N-(5-chloropentyl) derivative | cannabinoid | : | : | : | jwh018n5chlo |
| MAM-2201 chloropentyl derivative | cannabinoid | /sites/default/files/MAM-2201%2520N-(5-chloropentyl).png | 409 | 528 | mam2201chlo |
| UR-144 N-(5-chloropentyl) derivative | cannabinoid | /sites/default/files/UR-144%2520N-(5-chloropentyl).png | 391 | 458 | ur144n5chlo |
| JWH 018 N-(5-bromopentyl) derivative | cannabinoid | /sites/default/files/JWH-018%2520N-(5-bromopentyl).png | 391 | 528 | jwh018n5bro |
| AM-2232 | cannabinoid | /sites/default/files/AM-2232.png | 391 | 528 | am2232 |
| A-796,260 | cannabinoid | /sites/default/files/A-796_260.png | 350 | 487 | a796260 |
| JWH-200 | cannabinoid | /sites/default/files/JWH-200.png | 356 | 557 | jwh200 |
| WIN 48,098 / Pravadoline | cannabinoid | /sites/default/files/WIN%252048_098-pravadoline.png | 445 | 466 | win48098pr |
| AB-005 | cannabinoid | /sites/default/files/AB-005.png | 350 | 454 | ab005 |
| AM-1220 | cannabinoid | /sites/default/files/AM-1220.png | 356 | 524 | am1220 |
| AM-1248 | cannabinoid | /sites/default/files/AM-1248.png | 356 | 474 | am1248 |
| AM-2233 | cannabinoid | /sites/default/files/AM-2233.png | 356 | 433 | am2233 |
| JWH-250 1-(2-methylene-N-methyl-piperidyl) derivative | cannabinoid | /sites/default/files/JWH-250%2520Nmpm%2520deriv.png | 409 | 524 | jwh250nmpm |
| CRA-13 | cannabinoid | /sites/default/files/CRA-13.png | 457 | 469 | cra13 |
| JWH-307 | cannabinoid | /sites/default/files/JWH-307.png | 464 | 510 | jwh307 |
| JWH-370 | cannabinoid | /sites/default/files/JWH-370.png | 464 | 510 | jwh370 |
| JWH-368 | cannabinoid | /sites/default/files/JWH-368.png | 493 | 510 | jwh368 |
| JWH-307 bromine analogue | cannabinoid | /sites/default/files/JWH-307%2520bromine.png | 464 | 510 | jwh307bro |
| JWH-030 | cannabinoid | /sites/default/files/JWH-030.png | 310 | 510 | jwh030 |
| JWH-145 | cannabinoid | /sites/default/files/JWH-145.png | 464 | 510 | jwh145 |
| AM-6527 5F derivative | cannabinoid | /sites/default/files/AM-6527%25205F%2520derivative.png | 425 | 528 | am65275fderivative |
| AM-1248 azepane isomer | cannabinoid | /sites/default/files/AM-1248-azepane.png | 358 | 451 | am1248azepaneisomer |
| CUMYL | ring | /sites/default/files/CUMYL%2520R.gif | 207 | 110 | : |
| naphthyl | ring | /sites/default/files/naphthyl%2520core.png | 224 | 144 | : |
| adamantyl | ring | /sites/default/files/adamantyl%2520ring%2520system.png | 157 | 177 | : |
| cyclopropyl | ring | /sites/default/files/cyclopropyl%2520ring%2250system.png | 144 | 177 | : |
| quinolinyl | ring | /sites/default/files/quinolinyl%2520ring%2520system.png | 177 | 225 | : |
| phenyl | ring | /sites/default/files/phenyl%2520ring%2520system.png | 130 | 144 | : |
| 2-methyl (ring-substituent) | ringSubstituent | : | : | : | blank |
| 2-ethyl | ringSubstituent | : | : | : | blank |
| 2-methoxy | ringSubstituent | : | : | : | blank |
| 4-methoxy | ringSubstituent | : | : | : | blank |
| 2-iodo | ringSubstituent | : | : | : | :blank |
| 2-chloro | ringSubstituent | : | : | : | blank |
| 4-methyl | ringSubstituent | : | : | : | blank |
| 4-ethyl | ringSubstituent | : | : | : | blank |
| 4-propyl | ringSubstituent | : | : | : | blank |
| 4-fluoro | ringSubstituent | : | : | : | blank |
| 4-bromo | ringSubstituent | : | : | : | blank |
| 2,2,3,3-tetramethyl | ringSubstituent | : | : | : | blank |
| isopropyl | ringSubstituent | : | : | : | blank |
| tert-butyl | ringSubstituent | : | : | : | blank |
| methyl | tail | /sites/default/files/methylv2.png | 36 | 90 | : |
| propyl | tail | /sites/default/files/propyl.png | 83 | 171 | : |
| butyl | tail | /sites/default/files/butyl.png | 130 | 198 | : |
| pentyl | tail | /sites/default/files/pentyl2.png | 183 | 221 | |
| pent-4-enyl | tail | /sites/default/files/pentenyl%2520tail.png | 130 | 198 | : |
| pentoxy | tail | /sites/default/files/pentoxy%2520tail.png | 177 | 280 | : |
| 4-cyanobutyl | tail | /sites/default/files/4-cyanobutyl%2520T.gif | 156 | 78 | : |
| 5-fluoropentyl | tail | /sites/default/files/fluoropentyl%2520tail.png | 130 | 252 | : |
| 5-chloropentyl | tail | /sites/default/files/chloropentyl%2520tail.png | 130 | 252 | : |
| 5-bromopentyl | tail | /sites/default/files/bromopentyl%2520tail1.png | 130 | 252 | : |
| hexyl | tail | /sites/default/files/hexyl2.png | 177 | 280 | : |
| heptyl | tail | /sites/default/files/heptyl2.png | 177 | 279 | : |
| cyclohexylmethyl | tail | /sites/default/files/cyclohexylmethyl2.png | 177 | 198 | : |
| fluorobenzyl | tail | /sites/default/files/5-FLUOROBENZYL%2520T.gif | 225 | 125 | : |
| methylpiperidin-2-yl methyl | tail | /sites/default/files/methylpiperidin-2-yl%2520methyl%2520tail.png | 195 | 168 | : |
| methylazepan-3-yl | tail | /sites/default/files/methylazepan-3-yl%2520tail.png | 210 | 157 | : |
| tetrahydropyran-4-yl methyl | tail | /sites/default/files/tetrahydropyran-4-yl%2520tail.png | 195 | 139 | : |
| 2-morpholin-4-yl ethyl | tail | /sites/default/files/2-morpholin-4-yl%2520tail.png | 176 | 201 | : |
| indole | core | /sites/default/files/indole.png | 213 | 144 | : |
| indazole | core | /sites/default/files/Indazole%2520core.png | 213 | 144 | : |
| pyrrole | core | /sites/default/files/pyrrole%2520core.png | 124 | 120 | : |
| naphthalene | core | /sites/default/files/naphthylene%2520core.png | 224 | 144 | : |
| 2-methyl (core-substituent) | coreSubstituent | : | : | : | : |
| 5-phenyl | coreSubstituent | : | : | : | : |
| 5-(2-methyl)phenyl | coreSubstituent | : | : | : | : |
| 5-(2-fluoro)phenyl | coreSubstituent | : | : | : | : |
| 5-(2-bromo)phenyl | coreSubstituent | : | : | : | : |
| 5-(3-fluoro)phenyl | coreSubstituent | : | : | : | : |
| methanone | link | /sites/default/files/methanone.png | 117 | 130 | : |
| ethanone | link | /sites/default/files/ethanone.png | 144 | 130 | : |
| carboxamide | link | /sites/default/files/carboxamide%2520linker.png | 144 | 130 | : |
| carboxylate | link | /sites/default/files/carboxylate%2520linker.png | 144 | 130 | : |
| MDMB-CHMICA | cannabinoid | /sites/default/files/MDMB-CHMICA-new.png | 319 | 372 | mdmb-chmica |
| AB-PINACA | cannabinoid | /sites/default/files/AB-PINACAv2.gif | 295 | 414 | ab-pinaca |
| ADB-FUBINACA | cannabinoid | /sites/default/files/ADB-FUBINACAv2.gif | 331 | 387 | adb-fubinaca |
| ADB-PINACA | cannabinoid | /sites/default/files/ADB-PINACAv2.gif | 285 | 431 | adb-pinaca |
| AB-CHMINACA | cannabinoid | /sites/default/files/AB-CHMINACAv2.gif | 295 | 386 | ab-chminaca |
| ADB-CHMINACA | cannabinoid | /sites/default/files/ADB-CHMINACAv2.gif | 290 | 389 | adb-chminaca |
| 5F-MDMB-PINACA | cannabinoid | /sites/default/files/5F-MDMB-PINACA--5F-ADBv2.gif | 312 | 428 | 5f-mdmb-pinaca |
| MDMB-FUBINAC | cannabinoid | /sites/default/files/MDMB-FUBINACAv2.gif | 429 | 394 | mdmb-fubinaca |
| CUMYL-4CN-BINACA | cannabinoid | /sites/default/files/CUMYL-4CN-BINACAv2.gif | 294 | 525 | cumyl-4cn-binaca |
| MO-CHMINACA | cannabinoid | /sites/default/files/MO-CHMINACAv2.gif | 319 | 406 | mo-chminaca |
| carbamoyl | ring | /sites/default/files/Carbamoyl%2520R.gif | 192 | 186 | : |
| methoxycarbonyl | ring | /sites/default/files/methoxycarbonyl%2520R.gif | 255 | 184 | : |
Synthetic cannabinoid Apinaca
A synthetic cannabinoid that belongs to the adamantyl indazolecarboxamide family. It takes its codename from its systematic chemical name: N-(1-adamantyl)-1-pentyl-1H-indazole-3-carboxamide. It was first reported to the EMCDDA in May 2012 in Bulgaria when it was found in a smoking mixture product called ‘White Widow’. This substance also goes by the name ‘AKB-48’, the name of a popular all-girl band from Japan. This substance was critically reviewed by the WHO’s 36th Expert Committee on Drug Dependence in 2014.
Synthetic cannabinoid 5F-APINACA (5F-AKB48)
A synthetic cannabinoid of the adamantyl indazolecarboxamide family. It is chemically related to APINACA. It was first reported to the EMCDDA when it was detected in a herbal smoking mixture seized by Police in Latvia in September 2012. This substance was critically reviewed by the WHO’s 38th Expert Committee on Drug Dependence in 2016. It has been internationally controlled and will be included in Schedule II of the 1971 UN Convention on Psychotropic Substances.
Synthetic cannabinoid AM-2201 indazolecarboxamide analogue
A is a synthetic cannabinoid of the naphthyl indazolecarboxamide family. It was first reported to the EMCDDA in October 2012 by Finland where it was detected as a component in a white powder.
Synthetic cannabinoid Apica
A synthetic cannabinoid of the adamantyl indolecarboxamide family. It takes its codename from its systematic chemical name: N-(1-adamantyl)-1-pentyl-1H-indole-3-carboxamide. It was first reported to the EMCDDA in July 2012 and has been detected in bulk powders and in herbal smoking mixtures.
Synthetic cannabinoid JWH-018
A synthetic cannabinoid of the naphthoylindole family. It was first reported to the EMCDDA in December 2008 by Germany and Austria, being found as an ingredient in different varieties of ‘Spice’ products. JWH-018 is a controlled substance in many EU Member States. This substance is now internationally controlled and listed in Schedule II of the of the 1971 UN Convention on Psychotropic Substances.
Synthetic cannabinoid JWH-018 adamantoyl derivative
A synthetic cannabinoid of the adamantoylindole family. It was first reported to the EMCDDA in February 2011 when it was detected in branded herbal smoking mixtures such as ‘Nuclear Reactor’, ‘Toxic Waste’ and ‘Radio Active’. This substance also goes by the codename AB-001.
Synthetic cannabinoid AM-6527
A synthetic cannabinoid of the naphthyl indolecarboxamide family. It was first reported to the EMCDDA in July 2012 when it was detected by authorities in Finland. This substance has several codenames such as ‘MN24’, ‘NNIE’, ‘NNEI’, ‘NNE1’.
Synthetic cannabinoid PB-22
A synthetic cannabinoid of the quinolinyl indolecarboxylate family. It was first reported to the EMCDDA in November 2012 when it was detected by Finnish customs authorities in a seizure of 54 kilograms of light brown powder. PB-22 also goes by the codename ‘QUPIC’.
Synthetic cannabinoid JWH-022
A synthetic cannabinoid that belongs to the naphthoylindole family. This substance was first reported to the EMCDDA in November 2011 by the United Kingdom. It is normally found along with AM-2201 and it is known to be formed when AM-2201 breaks down metabolically and by thermal decomposition.
Synthetic cannabinoid AM-1220 Azepane Isomer
A synthetic cannabinoid that belongs to the naphthoylindole family. This substance was reported to the EMCDDA in May 2011. It is thought to be a by-product formed during the production of AM-1220.
Synthetic cannabinoid JWH-019
A synthetic cannabinoid receptor agonist that belongs to the naphthoylindole family. It was first reported to the EMCDDA in October 2010 by Finland. It has been found in herbal smoking mixtures and powders on its own and with other synthetic cannabinoids.
Synthetic cannabinoid BB-22
Little is known about this substance, a quinolinyl indolecarboxylate which shares some structural features similar to known synthetic cannabinoids. It was reported to the EMCDDA in January 2013 when it was detected in powders seized by Spanish authorities. BB-22 also goes by the codename ‘QUCHIC’.
Synthetic cannabinoid AM-2201
A synthetic cannabinoid that belongs to the naphthoylindole family. It was first reported to the EMCDDA in January 2011 by Latvian authorities and has been frequently reported ever since. Use of AM-2201 has been associated with convulsions. This substance is now internationally controlled and listed in Schedule II of the of the 1971 UN Convention on Psychotropic Substances.
Synthetic cannabinoid STS-135
A synthetic cannabinoid that belongs to the adamantyl indolecarboxamide family. It was first reported to the EMCDDA in June 2012 by Hungary and has been detected in powders and in branded herbal smoking mixtures such as ‘Armageddon’. STS-135 was the codename for the 135th mission of the American Space Shuttle programme.
Synthetic cannabinoid 5F-PB22
A synthetic cannabinoid of the quinolinyl indolecarboxylate family. This substance was first reported to the EMCDDA in March 2013 by Belgian authorities. Little is known about this novel compound.
Synthetic cannabinoid JWH 018 N-(5-chloropentyl) derivative
A synthetic cannabinoid that belongs to the naphthoylindole family. It was first reported to the EMCDDA by Germany in July 2012 and has been found often in combination with other synthetic cannabinoids in branded herbal smoking mixtures such as ‘Black Jack Silver’, ‘Black Jack Gold’, ‘New Bonzai Sommernight’ and ‘New Bonzai’.
Synthetic cannabinoid JWH 018 N-(5-bromopentyl) derivative
A synthetic cannabinoid that belongs to the naphthoylindole family. This brominated compound was reported to the EMCDDA by Germany in July 2012 when it was identified as one of the synthetic cannabinoids present in a herbal smoking mixture branded ‘XOXO’.
Synthetic cannabinoid AM-2232
A synthetic cannabinoid that belongs to the naphthoylindole family. It is the only synthetic cannabinoid monitored by the EMCDDA where the tail includes a nitrile group. It was first notified to the EMCDDA by Germany in December 2011 when it was identified as a component of a herbal smoking mixture branded ‘Summerlicious’.
Synthetic cannabinoid JWH-200
A synthetic cannabinoid that belongs to the naphthoylindole family. It was first reported to the EMCDDA in December 2009 when it was detected by authorities in Lithuania in a sample seized by border officials. It has since been detected in powders and in herbal smoking mixtures.
Synthetic cannabinoid AM-1220
A synthetic cannabinoid that belongs to the naphthoylindole family. It was first reported to the EMCDDA in May 2011 when it was detected by German authorities in a herbal smoking mixture branded ‘Soulman’.
Synthetic cannabinoid AM-1248
A cannabinoid receptor agonist of the adamantoylindole type. It was first reported to the EMCDDA in September 2012 when it was detected by German authorities in a herbal smoking mixture branded ‘Annihilation’.
Synthetic cannabinoid CRA-13
The first synthetic cannabinoid reported to the EMCDDA that belongs to the naphthoylnaphthalene family. It was reported in January 2011 by German authorities as a minor ingredient in a herbal smoking mixture. CRA-13 also goes by the codenames ‘CB-13’ and ‘SAB-378’.
Synthetic cannabinoid JWH-030
A synthetic cannabinoid of the naphthoylpyrrole family. It was reported to the EMCDDA in March 2013 by German authorities who detected it in a herbal smoking mixture also containing other (related) synthetic cannabinoids such as JWH-307 and JWH-145.
Synthetic cannabinoid A-834,735
A synthetic cannabinoid that belongs to the cyclopropylindole family. It was reported to the EMCDDA in January 2013 by Polish authorities who detected it in herbal smoking mixtures labelled ‘Sunny’ and ‘June Up’.
Synthetic cannabinoid AM-679
A synthetic cannabinoid that belongs to the benzoylindole family. It was reported to the EMCDDA in January 2012 by Italian authorities who detected it in a package of powder that was marked ‘AM XIAO’.
Synthetic cannabinoid JWH-081
A synthetic cannabinoid that belongs to the naphthoylindole family. It emerged in Europe in June 2010 when it was reported to the EMCDDA by Latvia, Germany, Finland, Austria and Norway. It is frequently detected in herbal smoking mixtures, often in combination with other synthetic cannabinoids.
Synthetic cannabinoid JWH-122
A synthetic cannabinoid receptor agonist that belongs to the naphthoylindole family. It was first reported to the EMCDDA in July 2010 by Latvian authorities. It is still present in the market and is often found as a component of herbal smoking mixtures containing multiple synthetic cannabinoids. It has been associated with intoxications in several countries.
Synthetic cannabinoid JWH-182
A synthetic cannabinoid that belongs to the naphthoylindole family. It was reported to the EMCDDA in February 2011 by Danish authorities. This is the only report of this substance in the context of the EU Early warning system.
Synthetic cannabinoid JWH-203
A synthetic cannabinoid that belongs to the phenylacetylindole family. It was first reported to the EMCDDA in October 2010 by Latvian authorities. It has been found in bulk powders and in branded herbal smoking blends such as ‘Aura Chrome’ and ‘Jah RUSH’.
Synthetic cannabinoid JWH-210
A synthetic cannabinoid that belongs to the naphthoylindole family. It was first reported to the EMCDDA in September 2010 by German authorities and has been detected regularly in bulk powders and in herbal smoking mixtures. Interestingly, it has been detected in herbal cannabis samples.
Synthetic cannabinoid JWH-250
A synthetic cannabinoid that belongs to the phenylacetylindole family. It was first reported to the EMCDDA in October 2009 by the German authorties and has remained in the market since then. It has been detected in bulk powders as well as in branded herbal smoking mixtures such as ‘Jamaican Gold’ and ‘Blast off’, frequently in combination with other synthetic cannabinoids. This substance was critically reviewed by the WHO’s 36th Expert Committee on Drug Dependence in 2014.
Synthetic cannabinoid JWH-251
A synthetic cannabinoid from the phenylacetylindole family. It was first reported to the EMCDDA in February 2011 by German authorities when it was the sole cannabimimetic detected in a branded herbal smoking mixture called ‘Aura Silver’.
Synthetic cannabinoid JWH-387
A synthetic cannabinoid belonging to the naphthoylindole family. This brominated compound was reported to the EMCDDA in July 2011 by German authorities who detected it in a white powder. This is the only report of this substance in the context of the Early warning system.
Synthetic cannabinoid JWH-398
A synthetic cannabinoid that belongs to the naphthoylindole family. It was first reported to the EMCDDA by the United Kingdom in October 2009 in 3 separate branded products, each time in combination with other cannabimimetic substances. It is not frequently reported to EMCDDA in the context of the EU Early warning system.
Synthetic cannabinoid JWH-412
A synthetic cannabinoid that belongs to the naphthoylindole family. It was reported to the EMCDDA in August 2011 by the German authorities, however, it has not been reported by any other countries in the context of the EU Early warning system.
Synthetic cannabinoid RCS-4
A synthetic cannabinoid that belongs to the benzoylindole family. The first formal notification to the EMCDDA was in July 2010 by Hungarian authorities, however, prior to this information had been received from Belarus regarding its detection. It is also known by the codenames ‘NRG-4’ and ‘DD001’. Other substances that have been detected with RCS-4 compounds are phenazepam and alphamethyltryptamine. This substance was critically reviewed by the WHO’s 36th Expert Committee on Drug Dependence in 2014.
Synthetic cannabinoid RCS-4 ortho isomer
A synthetic cannabinoid that belongs to the benzoylindole family. As the name suggests, it is closely related to RCS-4. It was first reported to the EMCDDA in April 2011 when it was detected in a sample of powder seized by Swedish authorities. Other substances that have been detected with RCS-4 compounds are phenazepam and alphamethyltryptamine.
Synthetic cannabinoid RCS-4 (C4)
A synthetic cannabinoid that belongs to the benzoylindole family. As the name suggests, it is closely related to RCS-4, differing only by the length of the alkyl ‘tail’. It was reported to the EMCDDA in June 2011 by Hungarian authorities who detected it in a mixture with RCS-4. Other substances that have been detected with RCS-4 compounds are phenazepam and alphamethyltryptamine.
Synthetic cannabinoid UR-144
A synthetic cannabinoid of the tetramethylcyclopropyl indolyl ketone family. It was first reported to the EMCDDA in February 2012 by Finland in a bulk powder and Poland in a branded herbal smoking mixture called ‘Magic Tree’. It acts as a selective agonist of the cannabinoid receptor CB2 and is often found in combination with other cannabimimetics. It is also known by the codenames ‘KM X-1’, ‘TMCP-018’, ‘MN-001’, ‘YX-17’. This substance was critically reviewed by the WHO’s 36th Expert Committee on Drug Dependence in 2014.
Synthetic cannabinoid JWH-122 pentenyl 2-methylindole derivative
A synthetic cannabinoid that belongs to the naphthoylindole family. Its first and only report to the EMCDDA was in July 2012 when it was detected in the United Kingdom in a sample that contained other cannabimimetic components. It is thought that this substance may be produced during the synthesis of MAM-2201.
Synthetic cannabinoid JWH-122 pentenyl derivative
A synthetic cannabinoid that belongs to the naphthoylindole family. The first report to the EMCDDA was in July 2012 when it was detected in the United Kingdom in a sample that contained other cannabimimetic components. It is thought that this substance may be produced during the synthesis of MAM-2201.
Synthetic cannabinoid UR-144 (-2H)
A synthetic cannabinoid of the tetramethylcyclopropyl indolyl ketone family. It was first reported to the EMCDDA in July 2012 by French authorities in branded herbal smoking mixtures called ‘Fire Ice’, ‘Pulse’, ‘Buzz’ and ‘Tribe’. It is thought that this substance may be produced during the synthesis of 5FUR-144.
Synthetic cannabinoid AB-005
A synthetic cannabinoid of the tetramethylcyclopropyl indolyl ketone family. It was first reported to the EMCDDA in November 2012 by German authorities. It was detected in a branded herbal smoking mixture called ‘Star of Fire’. The azepane isomer of AB-005 was also detected in this product.
Synthetic cannabinoid AB-005 azepane isomer
A synthetic cannabinoid of the tetramethylcyclopropyl indolyl ketone family. It was first reported to the EMCDDA in November 2012 by German authorities. It was detected in a branded herbal smoking mixture called ‘Star of Fire’ and is thought to be a by-product formed during the production of AB-005 (which was also found in the product).
Synthetic cannabinoid 3-(p-Methoxybenzoyl)-N-methylindole
A synthetic cannabinoid receptor agonist belongs to the benzoylindole family. The one and only report of this substance to the EMCDDA is from Austria in February 2012 when it was detected in a branded herbal smoking mixture called ‘Brooker Limited Edition’. It is thought that this substance is a chemical intermediate formed during the production of RCS-4.
Synthetic cannabinoid UR-144 heptyl derivative
A synthetic cannabinoid of the tetramethylcyclopropyl indolyl ketone family. It was first reported to the EMCDDA in April 2013 by Swedish authorities who detected it in a sample of white powder. It is thought that this substance will have similar properties to UR-144, as it differs only by the length of the alkyl ‘tail’.
Synthetic cannabinoid JWH-073
A synthetic cannabinoid belonging to the naphthoylindole family. It was first specifically reported to the EMCDDA by Denmark in March 2009 and has featured prominently in this market since then. It is similar to JWH-018, differing only in the length of the alkyl ‘tail’. It has been found in bulk powders, branded herbal smoking mixtures and also in resinous products. It is a controlled substances in many European countries. This substance was critically reviewed by the WHO’s 36th Expert Committee on Drug Dependence in 2014 and in 2016.
Synthetic cannabinoid JWH-073 methyl derivative
A synthetic cannabinoid belonging to the naphthoylindole family. It was first reported to the EMCDDA in April 2010 by German authorities who identified it in a branded herbal smoking mixture called ‘King B’. It is not frequently found, the only other instance being reported by Italian authorities in a sample that also contained JWH-073.
Synthetic cannabinoid 5FUR-144
A synthetic cannabinoid of the tetramethylcyclopropyl indolyl ketone family. It was first reported to the EMCDDA by the Latvian authorities in February 2012. It has been found in the form of bulk powders as well as in herbal smoking mixtures and in resinous products. It is also known by the codename ‘XLR-11’. This substance was critically reviewed by the WHO’s 38th Expert Committee on Drug Dependence in 2016. It has been internationally controlled and will be included in Schedule II of the of the 1971 UN Convention on Psychotropic Substances.
Synthetic cannabinoid AM-694
A synthetic cannabinoid that belongs to the benzoylindole family. It was first reported to the EMCDDA in July 2010 by the Irish authorities, having been detected in a herbal smoking product called ‘Shamrock’.
Synthetic cannabinoid AM-694 ethyl substituted for iodine
A synthetic cannabinoid that belongs to the benzoylindole family. As the name suggests, it is closely related to AM-694. It was reported to the EMCDDA in July 2012 in a sample of herbal smoking mixture from the United Kingdom that contained other derivatives of AM-694 and is thought to be a by-product of attempts at synthetic cannabinoid production.
Synthetic cannabinoid AM-694 methyl substituted for iodine
A synthetic cannabinoid that belongs to the benzoylindole family. As the name suggests, it is closely related to AM-694. It was reported to the EMCDDA in July 2012 in a sample of herbal smoking mixture from the United Kingdom that contained other derivatives of AM-694 and is thought to be a by-product of attempts at synthetic cannabinoid production.
Synthetic cannabinoid MAM-2201
A synthetic cannabinoid that belongs to the naphthoylindole family. It can be viewed as either a ring-methylated derivative of AM-2201 or an alkyl-fluorinated version of JWH-122. It was first reported to the EMCDDA in June 2011 by authorities in the Netherlands, but is currently a common ingredient of herbal smoking mixtures containing other synthetic cannabinoids. It has been reported to be associated with acute transient psychotic episodes.
Synthetic cannabinoid JWH-007
A synthetic cannabinoid that belongs to the naphthoylindole family. It was reported to the EMCDDA by German authorities in May 2011 having been detected in branded herbal smoking mixtures called “Sence” and “Oceanic Herbs”.
Synthetic cannabinoid EAM-2201
A synthetic cannabinoid that belongs to the naphthoylindole family. It can be viewed as either a ring-ethylated derivative of AM-2201 or an alkyl-fluorinated version of JWH-210. It was first reported to the EMCDDA in February 2013 by Swedish authorities in a sample of powder. It has also been detected in herbal smoking mixtures in combination with other synthetic cannabinoids.
Synthetic cannabinoid JWH-015
A synthetic cannabinoid receptor agonist that belongs to the naphthoylindole family. It has been reported to the EMCDDA only once, back in July 2010 when it was detected in a branded herbal smoking mixture called ‘Topaz’ by the authorities in Austria. The herbal material was identified as Damiana (Turnera diffusa).
Synthetic cannabinoid AM-694 chloro derivative
A synthetic cannabinoid that belongs to the benzoylindole family. As the name suggests, it is closely related to AM-694. It was reported to the EMCDDA in December 2011 by German authorities who detected it in a branded herbal smoking mixture called ‘Atomic Bomb’. The product also contained the parent molecule AM-694.
Synthetic cannabinoid MAM-2201 chloropentyl derivative
A synthetic cannabinoid that belongs to the naphthoylindole family. It can be viewed as the alkyl-chlorinated derivative of JWH-122. It was first reported to the EMCDDA in July 2012 in a sample of herbal smoking mixture from the United Kingdom that contained other derivatives of AM-2201 and is thought to be a by-product of attempts at synthetic cannabinoid production.
Synthetic cannabinoid UR-144 N-(5-chloropentyl) derivative
A synthetic cannabinoid of the tetramethylcyclopropyl indolyl ketone family, similar in structure to 5FUR-144. It was first reported to the EMCDDA in December 2012 by Hungarian authorities and subsequently in April 2013 by Croatian authorities. In each case, other cannabinoids were present including 5FUR-144./p>
Synthetic cannabinoid A-796,260
A synthetic cannabinoid of the tetramethylcyclopropyl indolyl ketone family. It is structurally related to UR-144 and to 5FUR-144. It has been reported to the EMCDDA on one occasion by Belgian authorities. It acts as a selective potent agonist of the cannabinoid receptor CB2.
Synthetic cannabinoid WIN 48,098/ Pravadoline
A synthetic cannabinoid that belongs to the benzoylindole family. It was detected in May 2011 by both the German and Polish authorities. It has been found in powders and in herbal smoking mixtures, sometimes in combination with other synthetic cannabinoids. It has been shown to be nephrotoxic in an animal model (dogs).
Synthetic cannabinoid JWH-145
A synthetic cannabinoid of the naphthoylpyrrole family. It was reported to the EMCDDA in March 2013 by German authorities who detected it in a herbal smoking mixture also containing other (related) synthetic cannabinoids such as JWH-307 and JWH-030.
Synthetic cannabinoid AM-2233
A synthetic cannabinoid that belongs to the benzoylindole family. It was first reported to the EMCDDA in August 2011 by Finnish authorities after it was detected in a seizure of white powder. It has also been detected in herbal smoking mixtures, on its own and in combination with other synthetic cannabinoids.
Synthetic cannabinoid JWH-250 1-(2-methylene-N-methyl-piperidyl) derivative
A synthetic cannabinoid that belongs to the phenylacetylindole family. It was first reported to the EMCDDA in March 2011 by Polish authorities. It was found in combination with JWH-122 in twenty herbal smoking mixtures such as ‘Red Mercury’, ‘Aztec Thunder’, ‘Zen Ultra’ and ‘Zephyr’.
Synthetic cannabinoid JWH-307
A synthetic cannabinoid that belongs to the naphthoylpyrrole family. It was first reported to the EMCDDA in August 2011 by authorities in Finland. It was detected in a seizure of powder. It has since been detected in several countries in various herbal smoking blends and in combination with other synthetic cannabinoids.
Synthetic cannabinoid JWH-307 bromine derivative
A synthetic cannabinoid that belongs to the naphthoylpyrrole family. It was reported to the EMCDDA in March 2013 by German authorities who detected it in a herbal smoking mixture also containing other (related) synthetic cannabinoids such as JWH-307 and JWH-030.
Synthetic cannabinoid JWH-368
A synthetic cannabinoid that belongs to the naphthoylpyrrole family. It was reported to the EMCDDA by Latvian authorities in February 2013 after it was detected in a bulk quantity of herbal mixture which also contained AM-2201.
Synthetic cannabinoid JWH-370
A synthetic cannabinoid that belongs to the naphthoylpyrrole family. It was first reported to the EMCDDA in February 2012 by Finnish authorities who detected it in a small sample of powder.
AM-6527 5F derivative
A synthetic cannabinoid of the naphthyl indolecarboxamide family. It was first reported to the EMCDDA in November 2013 when it was found in a herbal mixture with AM-6527 and MAM-2201.
AM-1248 azepane isomer
A synthetic cannabinoid belonging to the adamantoylindole family. Reported to hte EMCDDA in September 2013, it is thought to be a by-product formed during the production of AM-1248.
MDMB-CHMICA
An indolecarboxamide that contains a cyclohexylmethyl group. It was first reported to the EMCDDA in September 2014 by the Hungarian focal point when it was detected in a seizure of herbal material. MDMB-CHMICA has been associated with non-fatal intoxications and deaths in Europe. In July 2016, MDMB-CHMICA was risk-assessed by the EMCDDA and subsequently controlled throughout the EU, as of February 2017. The substance has been internationally controlled and will be placed in Schedule II of the of the UN 1971 Convention on Psychotropic Substances.
AB-PINACA
AB-PINACA is an indazolecarboxamide which is structurally related to Apinaca. This compound has also been identified in products sold in Japan. It was first reported to the EMCDDA in May 2013 by Sweden, when it was detected in an herbal mixture seized that also contained 5F-AKB48.
ADB-FUBINACA
ADB-FUBINACA is an indazolecarboxamide. It was first reported to the EMCDDA in November 2013 by the Turkish focal point. It was detected in herbal material seized containing also AB-PINACA and ADBICA. In 2015, tablets containing ADB-FUBINACA were associated with non-fatal intoxications in Hungary.
ADB-PINACA
ADB-PINACA is an an indazolecarboxamide which is structurally related to Apinaca. It was first reported to the EMCDDA in November 2013 by the United Kingdom focal point. ADB-PINACA was associated with an outbreak of non-fatal intoxications in the United States in September 2013.
AB-CHMINACA
AB-CHMINACA is an indazolecarboxamide. It was first reported to the EMCDDA in April 2014 by the Latvian focal point. AB-CHMINACA was detected in a seizure of plastic bags containing herbal material. The EMCDDA is monitoring intensively this substance.
ADB-CHMINACA
ADB-CHMINACA, also known as MAB-CHMINACA, is an indazolecarboxamide. It was first reported to the EMCDDA in September 2014 by the Hungarian focal point when it was detected in a seizure of powder. ADB-CHMINACA was associated with an outbreak of intoxications, including deaths, in the United States in 2015. The EMCDDA is monitoring intensively this substance.
5F-MDMB-PINACA
5F-MDMB-PINACA, also known as 5F-ADB, is an indazolecarboxamide. It was first reported to the EMCDDA in January 2015 by the Hungarian FP when it was detected in a seizure of powder. 5F-MDMB-PINACA has been associated with serious adverse events in Europe. The EMCDDA is monitoring intensively this substance.
MDMB-FUBINACA
MDMB-FUBINACA is an indazolecarboxamide. It was first reported to the EMCDDA in January 2016 by the Hungarian FP when it was detected in a seizure of powder. Products containing MDMB-FUBINACA in the Russian Federation were associated with an outbreak of serious adverse events in 2014.
CUMYL-4CN-BINACA
CUMYL-4CN-BINACA, also known as SGT-78, is an indazolecarboxamide that contains a cumyl group. It was first reported to the EMCDDA in February 2016 by the Hungarian FP when it was detected in a seizure of herbal material. CUMYL-4CN-BINACA has been associated with deaths in Europe. The EMCDDA is monitoring intensively this substance.
MO-CHMINACA
MO-CHMINACA, also known as MO-AMB, is an indazolecarboxamide, which is structurally related to MDMB-CHMICA. It was first reported to the EMCDDA in December 2016 by the Swedish focal point when it was detected in two biological samples.
Sorry…
No additional information on this cannabinoid is currently available. We are in the process of updating our information base and this should be available shortly.
Factos e números
- Mais de 620 novas substâncias psicoativas são atualmente monitorizadas pelo EMCDDA através do mecanismo de alerta rápido da UE, 169 das quais são agonistas dos recetores de canabinoides sintéticos.
- São conhecidas 14 famílias químicas de canabinoides sintéticos.
- 2008 — o JWH-018 foi o primeiro canabinoide sintético a ser detetado num produto «legal high»
A química e a denominação dos canabinoides sintéticos
Muitos dos canabinoides sintéticos monitorizados pelo EMCDDA através do mecanismo de alerta rápido da UE têm nomes de código relacionados com a sua descoberta, obtidos, em alguns casos, a partir das iniciais dos cientistas que os sintetizaram pela primeira vez: por exemplo, «JWH» corresponde a John W. Huffman e «AM» a Alexandros Makriyannis. Noutros casos, os códigos de nomes podem ter origem no nome da instituição ou empresa onde foram sintetizados pela primeira vez: a série «HU» de canabinoides sintéticos provém da Universidade Hebraica (Hebrew University) de Jerusalém, ou a série «CP» da Carl Pfizer. Em alguns casos, é provável que os nomes tenham sido escolhidos pelos fabricantes de produtos «legal high» para ajudar a comercializar os produtos. Exemplos claros desta opção são os nomes «AKB-48» e «2NE1», utilizados em alternativa às denominações APINACA e APICA. Na verdade, «AKB-48» é o nome de uma banda feminina japonesa muito popular e «2NE1» o de uma banda feminina da Coreia do Sul. Por último, o canabinoide sintético XLR-11 foi aparentemente batizado com o nome do primeiro foguetão de combustível líquido desenvolvido nos EUA para utilização em aeronaves, numa possível alusão ao que o vendedor promete aos consumidores da substância.
Muitos canabinoides sintéticos recebem agora nomes de código obtidos a partir das suas longas denominações químicas, por exemplo, APICA, de N-(1-adamantil)-1-pentil-1H-indole- 3-carboxamida, e APINACA, de N-(1-adamantil)-1-pentil-1H-indazole-3-carboxamida. O EMCDDA sistematizou este método tendo em vista a sua aplicação às novas substâncias emergentes e mostrar como as várias partes constituintes podem ser reunidas. As estruturas de muitos canabinoides sintéticos podem ser divididas em quatro categorias de componentes: cauda, núcleo, ligação e grupo ligado. A atribuição de um nome de código a cada componente permite identificar a estrutura química do canabinoide sem a sua longa denominação química. A sintaxe proposta para denominar os canabinoides sintéticos que obedecem a este padrão é a seguinte:
Grupoligado — CaudaNúcleoLigação
Esta ordenação dos componentes segue a ordem observada nas suas denominações químicas mais longas, como acontece em APICA: N-(1-adamantil)-1-pentil-1H-indole-3-carboxamida. Quando estiver presente um substituinte da cauda (isto é, 5F), deverá figurar à frente do nome e os substituintes do grupo ligado deverão figurar antes do grupo ligado; os substituintes do núcleo serão colocados no fim do código.
Aplicando o novo sistema a um canabinoide sintético recentemente notificado:
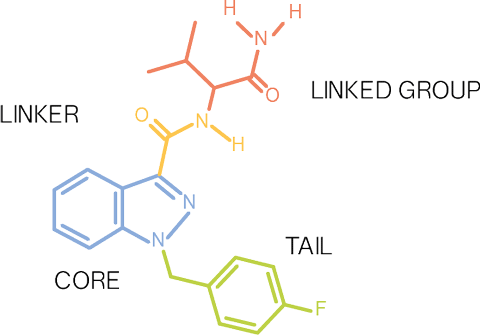
N-(1-carbamoil-2-metil-propil)-1-[(4-fluorofenil)metil] indazol-3-carboxamida
Denominação atual: AB-FUBINACA Nova denominação: MABO-FUBINACA
Os códigos de carateres utilizados baseiam-se não só nos carateres propriamente ditos, mas também na sua disposição. Por exemplo, «A» identifica a substância amina no grupo ligado; «CA» identifica a carboxamida. Aplicando a sintaxe e os códigos descritos, os canabinoides sintéticos que sigam esta estrutura terão uma abreviatura única.
- EMCDDA (2015), «Synthetic cannabinoids and “Spice” drug profile», sítio Web do EMCDDA.
- EMCDDA e Europol (2013), EU drug markets: A strategic analysis, Publicações Conjuntas do EMCDDA e da Europol, Serviço das Publicações da União Europeia, Luxemburgo.
- Gurney, S. M. R., Scott, K. S., Kacinko, S. L., Presley, B. C. e Logan, B. K. (2014), ‘Pharmacology, toxicology, and adverse effects of synthetic cannabinoid drugs’, Forensic Science Review 26, pp. 53–78.
- EMCDDA (2015), ‘New psychoactive substances in Europe. An update from the EU Early Warning System (March 2015)’, EMCDDA, Lisboa, março de 2015.
Find out more
Further reading
- EMCDDA (2015), «Synthetic cannabinoids and “Spice” drug profile», sítio Web do EMCDDA.
- EMCDDA e Europol (2013), EU drug markets: A strategic analysis, Publicações Conjuntas do EMCDDA e da Europol, Serviço das Publicações da União Europeia, Luxemburgo.
- Gurney, S. M. R., Scott, K. S., Kacinko, S. L., Presley, B. C. e Logan, B. K. (2014), ‘Pharmacology, toxicology, and adverse effects of synthetic cannabinoid drugs’, Forensic Science Review 26, pp. 53–78.
- EMCDDA (2015), ‘New psychoactive substances in Europe. An update from the EU Early Warning System (March 2015)’, EMCDDA, Lisboa, março de 2015.







