Synthetic cannabinoids and 'Spice' drug profile

About synthetic cannabinoids
Synthetic cannabinoids are functionally similar to Δ9-tetrahydrocannabinol (THC), the active principle of cannabis. Like THC, they bind to the same cannabinoid receptors in the brain and other organs as the endogenous ligand anandamide. More correctly designated as cannabinoid receptor agonists, they were initially developed over the past 40 years as therapeutic agents, often for the treatment of pain. However, it proved difficult to separate the desired properties from unwanted psychoactive effects.
In late 2008, several cannabinoids were detected in herbal smoking mixtures or so-called incense/room odorisers. Typical of these were Spice Gold, Spice Silver and Yucatan Fire, but many other products later appeared. They do not contain tobacco or cannabis but when smoked, produce effects similar to those of cannabis. These products are typically sold via the Internet and in ‘head shops’.
Chemistry
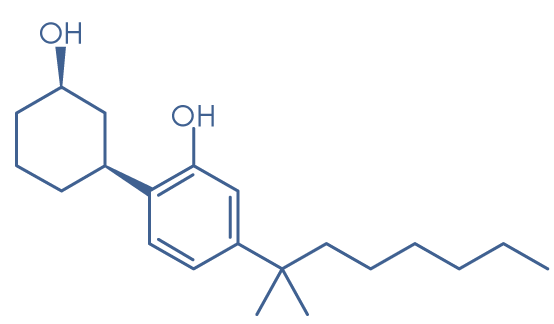
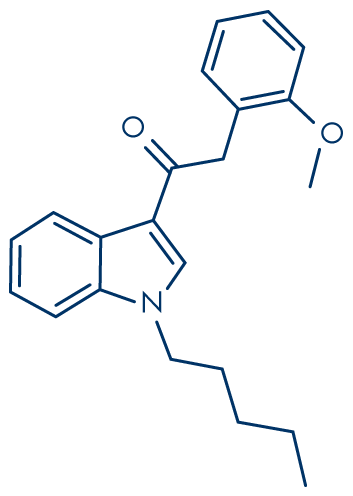
Although often referred to simply as synthetic cannabinoids, many of the substances are not structurally related to the so-called ‘classical’ cannabinoids, i.e. compounds, like THC, based on dibenzopyran. The cannabinoid receptor agonists form a diverse group, but most are lipid soluble and non-polar, and consist of 22 to 26 carbon atoms; they would therefore be expected to volatilize readily when smoked. A common structural feature is a side-chain, where optimal activity requires more than four and up to nine saturated carbon atoms. The first figure shows the structure of THC, while the others show examples of synthetic cannabinoid receptor agonists, all of which have been found in ‘Spice’ or other smoking mixtures. The synthetic cannabinoids fall into seven major structural groups:
- Naphthoylindoles (e.g. JWH-018, JWH-073 and JWH-398).
- Naphthylmethylindoles.
- Naphthoylpyrroles.
- Naphthylmethylindenes.
- Phenylacetylindoles (i.e. benzoylindoles, e.g. JWH-250).
- Cyclohexylphenols (e.g. CP 47,497 and homologues of CP 47,497).
- Classical cannabinoids (e.g. HU-210).
Other cannabinoid receptor agonists include substances such as oleamide — an endogenous substance that is also used in plastics manufacture — and methanandamide, both of which are structurally related to anandamide. However, the cannabinoid activity of these has been questioned. It is thought that neither methanandamide nor other arachidonyl derivatives related to anandamide would be sufficiently volatile to be smoked. Certain fluorosulfonates exhibit agonist activity at cannabinoid receptors, as does naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl)methanone, but the latter appears not to be psychoactive, at least when administered orally.
Structure of selected synthetic cannabinoids found in ‘Spice’ products, with high affinity for cannabinoid (CB1) receptors
Molecular structure: Δ9-THC
Molecular formula: C21H30O2
Molecular weight: 314.4 g/mol
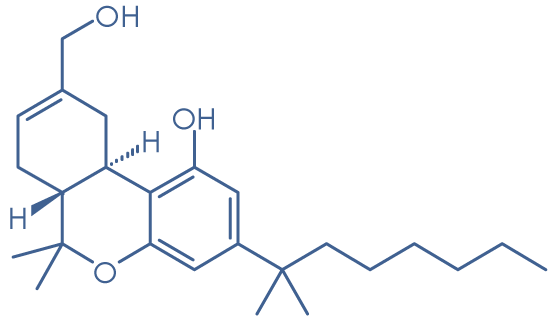
Molecular structure: HU-210
Molecular formula: C25H38O3
Molecular weight: 386.6 g/mol
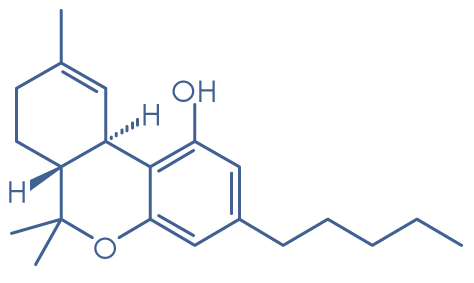
Molecular structure: CP 47,497
Molecular formula: C21H34O2
Molecular weight: 318.5 g/mol
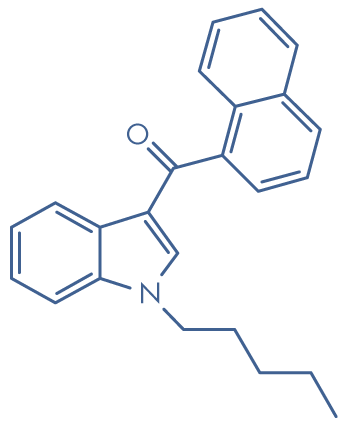
Molecular structure: JWH-018
Molecular formula: C24H23NO
Molecular weight: 341.5 g/mol
Molecular structure: JWH-250
Molecular formula: C22H25NO2
Molecular weight: 335.4 g/mol
Physical form
In the pure state, these substances are either solids or oils. Smoking mixtures are usually sold in metal-foil sachets, typically containing 3 g of dried vegetable matter to which one or more of the cannabinoids have been added. Presumably, a solution of the cannabinoids has been sprayed onto the herbal mixture. A number of plants are often listed on the packaging, but it appears that many are not present. However, large amounts of tocopherol (Vitamin E) have been detected, possibly to mask analysis of the active cannabinoids. The presence of several cannabinoids in some samples may also be intended to confound forensic-chemical detection.
Pharmacology
The cannabinoid receptor agonists mimic the effects of THC and anandamide by interacting with the CB1 receptor in the brain. In vitro studies have shown that some synthetic compounds bind more strongly to this receptor than THC as measured by the affinity constant Ki. All of the cannabinoids found in smoking mixtures have, like THC (Ki = 10.2nM), high affinity to the CB1 receptor although small variations in Ki values occur between different publications. The substance HU-210 has a particularly low value of Ki (0.06nM), and it binds over 100 times more tightly to the CB1 receptor than THC.
However, little is known about the detailed pharmacology and toxicology of the synthetic cannabinoids and few formal human studies have been published. It is possible that, apart from high potency, some cannabinoids could have particularly long half-lives potentially leading to a prolonged psychoactive effect. In addition, there could be considerable inter-and intra-batch variability in smoking mixtures, both in terms of substances present and their quantity. Thus, there is a higher potential for overdose than with cannabis.
Synthesis and precursors
Some cannabinoids are commercially available. Synthetic methods for many others have been published, while the precursor chemicals can often be purchased from retail chemical suppliers. However, synthesis of the naphthoylindoles from available starting materials requires many stages, whereas the preparation of dibenzopyrans is further complicated by the need to isolate the desired enantiomer from the racemic mixture.
Mode of use
Like cannabis, the herbal mixtures containing cannabinoids are most often smoked. However, some user reports also suggest that ‘Spice’ can be ingested as an infusion.
Other names
Herbal products containing synthetic cannabinoids have included Spice Gold, Spice Silver, Spice Diamond, Yucatan Fire, Sence, Chill X, Smoke, Genie, Algerian Blend and many others. These products may already be obsolete, since the Internet market is rapidly evolving; the synthetic cannabinoids used in the preparations are also being continuously substituted by ‘legal’ alternatives, in pace with new control measures.
Analysis
The cannabinoids are readily resolved using gas chromatography, but their identification and quantitative analysis is limited by the availability of pure reference samples. No field tests are known that will detect the majority of synthetic cannabinoids. Methods for the forensic analysis of blood samples for the recent intake detection of synthetic cannabinoids are available in some laboratories. However, the detection of metabolites in urine samples is not yet fully developed.
Typical purities
Few quantitative studies have been carried out to determine the amount of synthetic cannabinoids present in smoking mixtures. Because of the difficulty in making a homogeneous mixture of dried vegetable matter and small quantities of synthetic additives, it is likely that there could be considerable inter-batch differences in the concentration of cannabinoids.
Control status
None of the synthetic cannabinoids is under international control by virtue of the UN drug control conventions, but JWH-018, JWH-073, HU-210, and CP 47,497 (together with its C6, C8 and C9 homologues) are scheduled drugs in some Member States.
The following countries control ‘Spice’ and/or other synthetic cannabinoids: Denmark, Germany, Estonia, France, Ireland, Italy, Latvia, Lithuania, Luxembourg, Austria, Poland, Romania, Sweden and UK.
In Poland, JWH-018 and some of the claimed constituents of ‘Spice’ are controlled substances. In Germany, a fast-track regulation controls JWH-018 and CP 47,497. In Austria, Estonia and France, JWH-018, HU-210, and CP 47,497 are scheduled drugs; in addition to those, in Sweden and Lithuania JWH-073 is also classified as a narcotic. Luxembourg seems to have adopted an analogue approach by referring to ‘synthetic agonists of cannabinoid receptors’. The UK has adopted generic definitions and is expected to introduce control measures for a wide range of synthetic cannabinoids. Other Member Sates are also considering control measures.
Prevalence
Little is known about the extent to which smoking mixtures containing synthetic cannabinoids have replaced cannabis.
However, a 2009 survey conducted among 1 463 students aged between 15 and 18 years at schools providing general and vocational training in Frankfurt found that around 6 % of respondents reported having used ‘Spice’ at least once.
Street price
Sachets of smoking mixtures (3 g), sufficient for around eight joints, can be purchased for EUR 26 to 30 from Internet sites or specialist shops.
Medical use
Apart from THC (dronabinol), the only synthetic cannabinoid receptor agonist to have found clinical use is nabilone — a derivative of THC and constituent of the proprietary preparation Cesamet®; it finds limited use for the treatment of nausea in cancer chemotherapy.
Publications
Infographics and media
Bibliography
Aung, M. M., et al. (2000), ‘Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB1 and CB2 receptor binding’, Drug and Alcohol Dependence 60, pp. 133–140.
Auwärter, V., et al. (2009), ‘Spice and other herbal blends: harmless incense or cannabinoid designer drugs?’, Journal of Mass Spectrometry 44 (5), pp. 832–837.
Compton, D. R., et al. (1992), ‘Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents’, Journal of Pharmacology and Experimental Therapeutics 260 (1), pp. 201–209.
Compton, D. R., et al. (1993), ‘Cannabinoid structure–activity relationships: correlation of receptor binding and in vivo activities’, Journal of Pharmacology and Experimental Therapeutics 265 (1), p. 218.
Dziadulewicz, E. K., et al. (2007), ‘Naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl)methanone: a potent, orally bioavailable human CB1/CB2 dual agonist with antihyperalgesic properties and restricted central nervous system penetration’, Journal of Medicinal Chemistry 50, pp. 3851–3856.
De Vry, J. and Jentzsch, K. R. (2004), ‘Discriminative stimulus effects of the structurally novel cannabinoid CB1/ CB2 receptor partial agonist BAY 59-3074 in the rat’, European Journal of Pharmacology 505, pp. 127–133.
DEA (US Drugs Enforcement Administration) (2009), Microgram Bulletin (March), 42 (3).
Howlett, A. C., et al. (2002), ‘Classification of cannabinoid receptors’, International Union of Pharmacology XXVII, 54 (2), pp. 161–202.
Huffman, J. W. and Duncan, S. G. (1997), ‘Synthesis and pharmacology of the 1’,2’-dimethylheptyl-Δ8-THC isomers: exceptionally potent cannabinoids’, Bioorganic and Medicinal Chemistry Letters 7 (21), pp. 2799–2804.
Huffman, J. W., et al. (2003), ‘3-Indolyl-1-naphthylmethanes: new cannabimimetic indoles provide evidence for aromatic stacking interactions with the CB1 cannabinoid receptor’, Bioorganic and Medicinal Chemistry 11, pp. 539–549.
Huffman, J. W., et al. (2005), ‘Structure–activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB1 and CB2 receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB2 receptor agonists’, Bioorganic and Medicinal Chemistry 13, pp. 89–112.
Huffman, J. W., et al. (2005), ‘1-Pentyl-3-phenylacetylindoles: a new class of cannabimimetic indoles’, Bioorganic and Medicinal Chemistry Letters 15, pp. 4110–4113.
Huffman, J. W. (2009), ‘Cannabimimetic indoles, pyrroles, and indenes: structure–activity relationships and receptor interactions’, in Reggio, P. H. (ed.), The cannabinoid receptors, Humana Press, Totowa, NJ.
Lambert, D. and Di Marzo, V. (1999), ‘The Palmitoylethanolamide and oleamide enigmas: are these two fatty acid amides cannabimimetic?’, Current Medicinal Chemistry 6, pp. 757–773.
Lindigkeit, R., et al. (2009), ‘Spice: a never ending story?’, Forensic Science International 191(1–3), pp. 58–63.
Mauler, F., et al. (2002), ‘Characterization of the diarylether sulfonylester (-)-(R)-3-(2-Hydroxymethylindanyl-4-oxy)phenyl-4,4,4-trifluoro-1-sulfonate (BAY 38-7271) as a potent cannabinoid receptor agonist with neuroprotective properties’, Journal of Pharmacology and Experimental Therapeutics 302, pp. 359–368.
Mechoulam, R., et al. (1988), ‘Enantiomeric cannabinoids: stereospecificity of psychotropic activity’, Experientia 44, pp. 762–764.
Pertwee, R. G. (2005), ‘Pharmacological actions of cannabinoids’, in Pertwee, R. (ed.), Cannabinoids, Springer, Berlin.
Piggee, C. (2009), ‘Investigating a not-so-natural high’, Analytical Chemistry 81 (9), pp. 3205–3207.
Steup, C. (2008), ‘Untersuchung des Handelsproduktes “Spice”’, 30 December, THC Pharm GmbH.
Uchiyama, N., et al. (2009), ‘Identification of a cannabinoid analog as a new type of designer drug in a herbal product’, Chemical and Pharmaceutical Bulletin 57 (4), pp. 439–441.
Uchiyama, N., et al. (2009), ‘Identification of a cannabimimetic indole as a designer drug in a herbal product’, Forensic Toxicology 27, pp. 61–66.
Vann, R.E., et al. (2009), ‘Discriminative stimulus properties of Δ9-tetrahydrocannabinol (THC) in C57BL/6J mice’, European Journal of Pharmacology 615 (1–3), pp. 102–107.
Weissman, A., et al. (1982), ‘Cannabimimetic activity from CP-47,497, a derivative of 3-phenylcyclohexanol’, Journal of Pharmacology and Experimental Therapeutics 223 (2), pp. 516–23.
Wiley, J. L., et al. (1998), ‘Structure–activity relationships of indole- and pyrrole-derived cannabinoids’, Journal of Pharmacology and Experimental Therapeutics 285 (3), pp. 995–1004.
Zimmermann, U. S., et al. (2009), ‘Withdrawal phenomena and dependence syndrome after the consumption of “Spice Gold”’, Deutsches Aerzteblatt International 106 (27), pp. 464–467.



















