Synthetic cathinones drug profile

About Synthetic cathinones
Synthetic cathinones are related to the parent compound cathinone (Figure 1), one of the psychoactive principals in khat (Catha edulis Forsk). Cathinone derivatives are the β-keto (βk) analogues of a corresponding phenethylamine. The group includes several substances that have been used as active pharmaceutical ingredients (API) of medicinal products, e.g. amfepramone (diethylpropion; Figure 2). Since the mid-2000s, unregulated ring-substituted cathinone derivatives have appeared in the European recreational drugs market. The most commonly available cathinones sold on the recreational market in the period up to 2010 appear to be mephedrone (Figure 3) and methylone (Figure 4). These products are usually encountered as highly pure white or brown powders. Ring-substituted cathinone derivatives are claimed to have effects similar to those of cocaine, amphetamine or MDMA (ecstasy), but little is known of their detailed pharmacology. Apart from cathinone (Figure 1), methcathinone (Figure 5) and two API’s amfepramone (Figure 2) and pyrovalerone, cathinone derivatives are not under international control.
Chemistry
Figures 1–7 show the naturally occurring cathinone (Figure 1) and six synthetic derivatives (Figures 2–7).
Figure 1: Cathinone
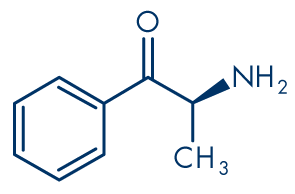
Molecular formula: C9H11NO
Molecular weight: 149.19 g/mol
Figure 2: Amfepramone (diethylpropion)
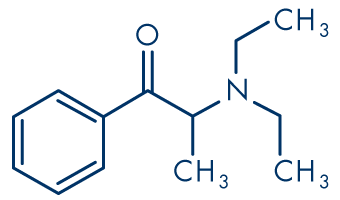
Molecular formula: C13H19NO
Molecular weight: 205.30 g/mol
Figure 3: Mephedrone (4-methylmethcathinone, 4-MMC)
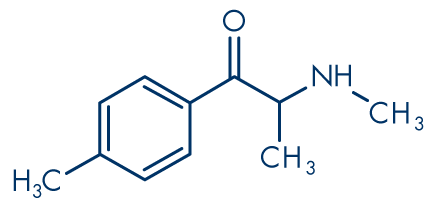
Molecular formula: C11H15NO
Molecular weight: 177.24 g/mol
Figure 4: Methylone (βk-MDMA, 3,4-methylenedioxy-N-methylcathinone)
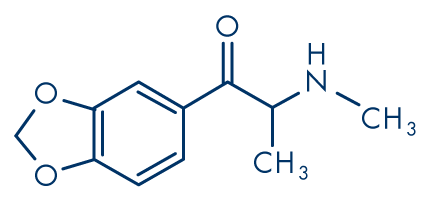
Molecular formula: C11H13NO3
Molecular weight: 207.22 g/mol
Figure 5: Methcathinone (ephedrone)

Molecular formula: C10H13NO
Molecular weight: 163.22 g/mol
Figure 6: MDPV (3,4-methylenedioxypyrovalerone)

Molecular formula: C16H21NO3
Molecular weight: 275.35 g/mol
Figure 7: Methedrone (βk-PMMA, 4-methoxymethcathinone)
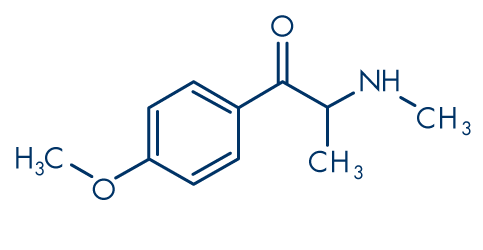
Molecular formula: C11H15NO2
Molecular weight: 193.24 g/mol
Cathinone (Figure 1) and its derivatives are closely related to the phenethylamine family. Thus cathinone itself is β-keto (βk) amphetamine, 2-aminopropiophenone or, more formally, 2-amino-1-phenyl-1-propanone (IUPAC systematic name). The first synthetic cathinone that appeared in the recreational drug market, methcathinone (Figure 5), is βk-methamphetamine, ephedrone or N-methylcathinone. Most of the unregulated cathinone derivatives that have been marketed in the past few years are ring-substituted, the most prevalent of which appears to be mephedrone (4-methylmethcathinone, 4-MMC, Figure 3). Some products sold are also likely to contain a mixture of different chemicals. Other cathinones reported to the Early warning system on new drugs include methylone (βk-MDMA; 3,4-methylenedioxy-N-methylcathinone, Figure 4), MDPV (3,4-methylenedioxypyrovalerone, Figure 6), methedrone (βk-PMMA; 4-methoxymethcathinone, Figure 7) and PPP (α-pyrrolidinopropiophenone).
Like the phenethylamines, cathinone derivatives can exist in two stereoisomeric forms, which may differ in their potencies. The cathinone that occurs naturally in khat is the S-enantiomers. However, it is likely that most ring-substituted derivatives are racemic mixtures. It is also believed that racemisation of all cathinone derivatives can occur through keto-enol tautomerism. Cathinone is labile and transforms to a dimer (3,6-dimethyl-2,5-diphenylpyrazine). Cathinone derivatives can also rearrange via a dihydropyrazine dimer to form so-called ‘isocathinones’; All known cathinone derivatives are either N-alkylated or the nitrogen atom is part of a pyrrolidine ring, and most are produced as hydrochloride salts. Many illicit products are N-methylated, i.e. ephedrone derivatives, whereby mephedrone can be described as 4-methylephedrone. The pyrrolidine derivatives (PPP, MDPV) can be regarded as a sub-set of ‘designer drugs’ sharing the same skeleton as pyrovalerone. Table 1 lists those cathinone derivatives that have been used as API, found in drug seizures, samples collected for monitoring purposes or offered for sale on Internet sites (see Figure 8). Naphyrone (1-naphthalen-2-yl-2-pyrrolidin-1-ylpentan-1-one), a more complex cathinone derivative, is not included in Table 1.
Figure 8: General structure of a cathinone derivative showing substitution patterns
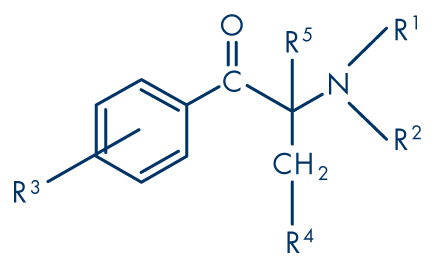
Table 1
The structural classification of cathinone derivatives found in drug seizures, collected samples or offered for sale on Internet sites (see Figure 8). Those that are or have been used as active pharmaceutical ingredients (API) are shown emboldened.
| R1 | R2 | R3 | R4 | R5 | Name |
| H | H | H | H | H | Cathinone |
| Methyl | H | H | H | H | Methcathinone (ephedrone) |
| Methyl | Methyl | H | H | H | N,N-Dimethylcathinone (metamfepramone) |
| Ethyl | H | H | H | H | N-Ethylcathinone (EC) |
| Methyl | H | H | Methyl | H | Buphedrone |
| Ethyl | H | 4-Methyl | H | H | 4-Methyl-N-ethylcathinone |
| Methyl | H | 4-Methyl | H | H | Mephedrone (4-MMC; M-CAT) |
| Ethyl | Ethyl | H | H | H | Amfepramone |
| t-Butyl | H | 3-CI | H | H | Bupropion |
| Methyl | H | 3,4-Methylenedioxy | H | H | Methylone (βk-MDMA) |
| Ethyl | H | 3,4-Methylenedioxy | H | H | Ethylone (βk-MDEA) |
| Methyl | H | 4-Methyl | Methyl | H | Butylone (βk-MBDB) |
| Methyl | H | 4-Methoxy | H | H | Methedrone (βk-PMMA) |
| Methyl | H | 4-F | H | H | Flephedrone (4-FMC) |
| Methyl | H | 3-F | H | H | 3-Fluoromethcathinone (3-FMC) |
| {pyrrolidino} | H |
H |
H | α-Pyrrolidinopropiophenone (PPP) | |
| {pyrrolidino} | 4-Methyl | H | H | 4-Methyl-α-pyrrolidinopropiophenone (MPPP) | |
| {pyrrolidino} | 4-MeO | H | H | 4-methoxy-α-pyrrolidinopropiophenone (MOPPP) | |
| {pyrrolidino} | 4-Methyl | Propyl | H | 4-Methyl-α-pyrrolidino-hexanophenone (MPHP) | |
| {pyrrolidino} | 4-Methyl | Ethyl | H | Pyrovalerone | |
| {pyrrolidino} | 4-Methyl | Methyl | H | 4-Methyl-α-pyrrolidino-butyrophenone (MPBP) | |
| {pyrrolidino} | 4-Methyl | H | Methyl | 4-Methyl-α-pyrrolidino-α-methylpropiophenone | |
| {pyrrolidino} | 3,4-Methylenedioxy | H | H | 3,4-Methylenedioxy-α-pyrrolidinopropiophenone (MDPPP) | |
| {pyrrolidino} | 3,4-Methylenedioxy | Ethyl | H | 3,4-Methylenedioxypyrovalerone (MDPV) | |
Physical form
Synthetic cathinones are mostly encountered as white or brown amorphous or crystalline powders, occasionally encapsulated. Unlike many phenethylamine derivatives (MDMA, etc.), tablets are less common but are sometimes available on the illicit market, presumably as a replacement for MDMA.
Pharmacology
As with phenethylamines, in the absence of ring-substitution, cathinones behave as central nervous system (CNS) stimulants, although invariably with a lower potency than the corresponding phenethylamine analogue. The lower potency is caused by the β-keto group creating a more polar molecule less able to cross the blood–brain barrier. Studies on the metabolism of methcathinone derivatives in rats and humans have shown that they are N-demethylated, the keto group is reduced to hydroxyl, and ring alkyl groups are oxidised. Otherwise, few formal studies have been made on the pharmacokinetics or pharmacodynamics of ring-substituted cathinones. From observations of patients who presented with suspected mephedrone toxicity, it appears that cathinone derivatives show similar sympathomimetic effects to amphetamine derivatives. The first toxicologically confirmed fatal case directly linked to mephedrone use was recorded in Sweden in 2008.
User reports on Internet sites suggest that a typical dose of mephedrone is 100–250 mg. Depending on the particular substance, the effects are claimed to be similar to those of cocaine, amphetamine or MDMA. Like cocaine, the resulting ‘high’ of mephedrone is short-lived. Consequently, users may consume several doses in succession, up to 1 g in a session. This is supported by the finding that the most common ‘wrap size’ of mephedrone found in police seizures in the United Kingdom is close to 750 mg.
The pyrrolidine ring and the tertiary amino group in MDPV could lead to a more lipophilic, i.e. more potent, molecule; Internet user-forums suggest that the dose is as low as 5–10 mg. Furthermore, it should be noted that p-methoxyphenethylamines (e.g. PMA, PMMA) are known to have a particularly high toxicity, and this property might translate to their βk-analogues. For example, methedrone (p-methoxymethcathinone) has been detected in a few fatalities.
Synthesis and precursors
Simple derivatives such as methcathinone and N,N-dimethylcathinone can be synthesised by oxidation of ephedrine (or pseudoephedrine) and N-methylephedrine (or N-methylpseudoephedrine) respectively. This requires reacting the precursor with a solution of potassium permanganate in dilute sulfuric acid. The precursors can be obtained as specific enantiomers, thereby ensuring that the synthesis is stereoselective. Cathinone itself can be made in a similar way, starting from phenylpropanolamine (norephedrine). One of the hazards of the permanganate process is that users can suffer manganese poisoning if the product is not purified.
The ring-substituted N-methylcathinone derivatives are best synthesised by reacting the suitably substituted bromopropiophenone with methylamine; the result is always racemic. In the case of methylone, for example, 2-bromo-3,4-methylenedioxy-propiophenone can be prepared by reacting 3,4-methylenedioxypropiophenone with bromine. These precursor substances are readily available and none of them is under international control. Other methods are required to produce the pyrrolidine derivatives, but apart from MDPV, substances such as PPP, MPHP, MOPPP and MDPPP, which briefly appeared in Germany in 2004 (see Table 1), have since been rarely observed.
Mode of use
Some users insufflate (snort) mephedrone, but most of the cathinones are ingested. Since they are soluble in water, these substances can also be injected. Because of their lability, the free bases would probably not be suitable for smoking.
Other names
To circumnavigate possible controls, suppliers of cathinone derivatives often market them under various brand names (e.g. Explosion, Blow, Recharge, etc.) as ‘plant food’, ‘bath salts’, or ‘research chemicals’, often with a printed warning that they are ‘not for human consumption’. As with the phenethylamines, acronyms are common. Thus MDPV stands for 3,4-methylenedioxypyrovalerone, 4-FMC for 4-fluoromethcathinone (flephedrone), and 4-MMC for 4-methylmethcathinone (mephedrone). User names for mephedrone include M-Cat, meph, drone, miaow, meow meow, subcoca-1 and bubbles; while methylone is sometimes known as Top Cat. However, these substances are often sold in products that have a large number of brand names that change rapidly over time and where the specific content is often not given. The chemical names can lead to confusion; methylone, mephedrone and methedrone should be distinguished from each other and from the unrelated narcotic analgesic methadone. Although βk-MBDB is often described as ‘butylone’, butylone has also been used as a proprietary name for the unrelated barbiturate pentobarbital.
Analysis
Cathinone derivatives do not give a coloured reaction with the Marquis field test. Analysis using GCMS and Infrared (IR) spectroscopy is straightforward. Although pure reference samples of some derivatives may not be commercially available, analytical profiles for most have been published. Immunoassay field tests for methamphetamine give false positive reactions with some cathinone derivatives.
Typical purities
Some powders containing mephedrone and related compounds have been adulterated with other drugs such as ketamine, cocaine, paracetamol or piperazine derivatives, but most appear to be highly pure as judged by IR spectroscopy.
Control status
Cathinone and methcathinone are listed in Schedule I of the United Nations 1971 Convention on Psychotropic Substances. Amfepramone and pyrovalerone are in Schedule IV of that Convention, but other derivatives are not under international control. A few cathinone derivatives are controlled in some Member States under drug control or equivalent legislation, for example: mephedrone (Belgium, Denmark, Germany, Estonia, Ireland, France, Italy, Lithuania, Romania, Sweden, Croatia and Norway); methylone (Denmark, Ireland, Romania and Sweden); butylone (Denmark, Ireland, Romania, Sweden and Norway); MDPV (Denmark, Ireland, Finland and Sweden); and flephedrone (Denmark, Ireland and Romania). Generic control in the United Kingdom covers a wide group of cathinone derivatives. Mephedrone is controlled under medicines legislation in Finland and the Netherlands and Finland.
By Council Decision of 2 December 2010, 4-methylmethcathinone (mephedrone) was submitted to control measures in EU Member States (2010/759/EU).
Medical use
Amfepramone and pyrovalerone have been used as anorectics, but are now largely obsolete. Bupropion has antidepressant properties and is used as an aid for those who wish to quit tobacco smoking.
Publications
Bibliography
Advisory Council on the Misuse of Drugs (2010) Consideration of the cathinones, 31 March. Advisory Council on the Misuse of Drugs, Home Office, London. Available at: http://www.homeoffice.gov.uk/publications/drugs/acmd1/acmd-cathinodes-r….
Archer, R. (2009) ‘Fluoromethcathinone: A new substance of abuse’, Forensic Science International 185 (1), pp. 10–20.
Belal, T., Awad, T., De Ruiter, J. and Clark, C. R. (2009) ‘GC–IRD methods for the identification of isomeric ethoxyphenethylamines and methoxymethcathinones’, Forensic Science International 184, pp. 54–63.
Bossong, M. G., Van Dijk, J. P. and Niesink ,R. J. (2005) ‘Methylone and mCPP: Two new drugs of abuse?’ Addiction Biology 10 (4), pp. 321–323.
Camilleri, A., Johnston, M. R., Brennan, M., Davis, S. and Caldicott, D. G. E. (2010) ‘Chemical analysis of four capsules containing the controlled substance analogues 4-methylmethcathinone, 2-fluoromethamphetamine, alpha-phthalimidopropiophenone and N-ethylcathinone’, Forensic Science International 197 (1–3), pp. 59–66.
Carroll, F. Y., Blough, B. E., Mascarella, S. W., et al. (2010) ‘Synthesis and biological evaluation of bupropion analogues as potential pharmacotherapies for smoking cessation’, Journal of Medicinal Chemistry 53 (5), pp. 2204–2214.
Chintalova-Dallas, R., Case, P., Kitsenko, N. and Lazzarini, Z. (2009) ‘Boltushka: A homemade amphetamine-type stimulant and HIV risk in Odessa, Ukraine,’ International Journal of Drug Policy 20, pp. 347–351.
Cozzi, N. V., Shulgin, A. T. and Ruoho, A. E. (1998) ‘Methcathinone (MCAT) and 2-methylamino-1-(3,4-methylenedioxyphenyl)propan-1-one (MDMCAT) inhibit [3H]serotonin uptake into human platelets’, American Chemical Society Division of Medicine Abstracts 215, p. 152.
Cozzi, N. V., Sievert, M. K., Shulgin, A. T., Jacob III, P. and Ruoho, A. E. (1998) ‘Methcathinone and 2-methylamino-1-(3,4-methylenedioxyphenyl)propan-1-one (methylone) selectively inhibit plasma membrane catecholamine reuptake transporters’, Society for Neuroscience Abstracts 24, pp. 381–388.
Cozzi, N. V. Sievert, M. K., Shulgin, A.T., Jacob III, P. and Ruoho, A. E. (1999) ‘Inhibition of plasma membrane monoamine transporters by β-ketoamphetamines’, European Journal of Pharmacology 381, pp. 63–69.
Dal Cason, T. A. (1997) ‘The characterization of some 3,4-methylenedioxycathinone (MDCATH) homologs’, Forensic Science International 87, pp. 9–53.
Dal Cason, T. A. (2007) ‘Synthesis and identification of N,N-dimethylcathinone hydrochloride’ Microgram Journal 5 (page 1–4). Available at: http://www.justice.gov/dea/programs/forensicsci/microgram/journal_v5_num14/pg1.html.
Daly, M. (2010) ‘Teenage kicks’, Druglink 25 (1), pp. 8–10.
Davies, S., Archer, R. and Ramsey, J. (2009) Analytical profiles of methcathinone and related compounds, London Toxicology Group, London. Available at: http://www.ltg.uk.net/admin/files/Methcath.pdf.
Davies, S., Puchnarewicz, M., Button, J., et al. (2009) ‘Two cases of confirmed ingestion of the novel designer compounds: 4-methylmethcathinone (mephedrone) and 3-fluoromethcathinone’, London Toxicology Group poster, London. Available at: http://www.ltg.uk.net/admin/files/MethCase(2).pdf.
Dickson, A. J., Vorce, S. P., Levine, B. and Past, M. R. (2010) ‘Multiple-drug toxicity caused by the coadministration of 4-methylmethcathinone (mephedrone) and heroin’, Journal of Analytical Toxicology 34 (3), pp. 162–168.
Gibbons, S. and Zloh, M. (2010) ‘An analysis of the “legal high” mephedrone’, Bioorganic & Medicinal Chemistry Letters 20 (14), pp. 4135–4139.
Gustavsson, D. and Escher, C. (2009) ‘Mefedron: Internetdrog som tycks ha kommit för att stanna’, Läkartidningen 106 (43), pp. 2769–2771. English summary available at: http://www.lakartidningen.se/07engine.php?articleId=12986.
Kamata, H. T., Shima, N., Zaitsu, K. et al. (2006) ‘Metabolism of the recently encountered designer drug, methylone, in humans and rats’, Xenobiotica 36 (8), pp. 709–723.
Measham, F., Moore, K., Newcombe, R. and Welch, Z. (2010) ‘Tweaking, bombing, dabbing and stockpiling: The emergence of mephedrone and the perversity of prohibition’, Drugs and Alcohol Today, 2010, 10 (pages 14-21).
Meltzer, P. C., Butler, D., Deschamps, J. R. and Madras, B. K. (2006) ‘1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (pyrovalerone) analogues: A promising class of monoamine uptake inhibitors’, Journal of Medicinal Chemistry 49, pp. 1420–1432.
Meyer, M. R., Peters, F. T. and Maurer, H. H. (2009) ‘Metabolism of the new designer drug mephedrone and toxicological detection of the beta keto designer drugs mephedrone, butylone and methylone in urine’, Annales de Toxicologie Analytique 21.
Meyer, M. R., Wilhelm, J., Peters, F. T. and Maurer, H. H. (2010) ‘Beta-keto amphetamines: Studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography–mass spectrometry’, Analytical and Bioanalytical Chemistry 397 (3), pp. 1225–1233.
Nagai, F., Nonaka, R. and Kamimura, K. S. H. (2007) ‘The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain’, European Journal of Pharmacology 559 (2–3), pp. 132–137.
Naoya, A., Kiyoshi, W. and Masahiko, F. (2006) ‘Role of dopaminergic system in the expression of methylone-induced behavioral effect in mice’. Bulletin of the Japanese Society for Neurochemistry 45(2–3), pp. 301.
Power, M. (2010) ‘Mephedrone: The future of drug dealing?’, Druglink March/April 2010, Volume 25(1), pp. 7–13.
Power, M. (2010) ‘World wired web’, Druglink 25 (1), pp. 11–13.
Roussel, O., Perrin, M., Herard, P., Chevance, M. and Arpino, P. (2009) ‘La 4-méthyléphédrone sera-t-elle une “Ecstasy” du XXIème siècle?’, Annales de Toxicologie Analytique 21 (4), pp. 169–177.
Shima, N., Katagi, M. and Tsuchihashi, H. (2009) ‘Direct analysis of conjugate metabolites of methamphetamine, 3,4-methylenedioxymethamphetamine, and their designer drugs in biological fluids’, Journal of Health Science 55 (4), pp. 495–502.
Shimizu, E., Watanabe, H., Kojima, T. et al. (2007) ‘Combined intoxication with methylone and 5-MeO-MIPT’, Progress in Neuro-Psychopharmacology and Biological Psychiatry 31 (1), pp. 288–291.
Springer, D., Fritschi, G. and Maurer, H. H. (2003) ‘Metabolism of the new designer drug α-pyrrolidinopropiophenone (PPP) and the toxicological detection of PPP and 4'-methyl-α-pyrrolidinopropiophenone (MPPP) studied in rat urine using gas chromatography-mass spectrometry’, Journal of chromatography. Analytical technologies in the biomedical and life sciences 796 (2), pp. 53–66.
Springer, D., Fritschi, G. and Maurer, H. H. (2003) ‘Metabolism and toxicological detection of the new designer drug 3',4'-methylenedioxy-α-pyrrolidinopropiophenone studied in urine using gas chromatography-mass spectrometry’, Journal of chromatography. Analytical technologies in the biomedical and life sciences 793 (2), pp. 377–388.
Springer, D., Fritschi, G. and Maurer, H. H. (2003) ‘Metabolism and toxicological detection of the new designer drug 4'-methoxy-α-pyrrolidinopropiophenone studied in rat urine using gas chromatography-mass spectrometry’, Journal of chromatography. Analytical technologies in the biomedical and life sciences 793 (2), pp. 331–342.
Springer, D., Peters, F.T., Fritschi, G. and Maurer, H. H. (2003) ‘New designer drug 4′-methyl-α-pyrrolidinohexanophenone: Studies on its metabolism and toxicological detection in urine using gas chromatography–mass spectrometry’, Journal of chromatography. Analytical technologies in the biomedical and life sciences 789 (1), pp. 79–91.
Springer, D., Staack, R. F., Paul, L. D., Kraemer, T. and Maurer, H. H. (2003) ‘Identification of cytochrome P450 enzymes involved in the metabolism of 4-methoxy-a-pyrrolidinopropiophenone (MOPPP), a designer drug, in human liver microsomes’, Xenobiotica 33 (10), pp. 989–998.
Staack, R.F, .Fritschi, G. and Maurer, H. H. (2002) ‘Studies on the metabolism and toxicological detection of the new designer drug 4'-methyl-a-pyrrolidinopropiophenone in urine using gas chromatography-mass spectrometry’, Journal of chromatography. Analytical technologies in the biomedical and life sciences773 (1), pp. 25–33.
Sumnall, H. and Wooding, O. (2010) Mephedrone: An update on current knowledge, North West Public Health Laboratory, Liverpool John Moores University. Available at: http://www.cph.org.uk/showPublication.aspx?pubid=614.
Szendrei, K. (1980) ‘The chemistry of khat’, Bulletin on Narcotics 32 (3), pp. 5–35.
Uchiyama, N., Kikura-Hanajiri, R., Kawahara, N., and Goda, Y. (2008) ‘Analysis of designer drugs detected in the products purchased in fiscal year 2006’, Journal of the Pharmaceutical Society of Japan 128 (10), pp. 1499–1506.
Westphal, F., Junge, T., Rösner, P., et al. (2006) ‘Mass spectral and NMR spectral data of two new designer drugs with an α-aminophenone structure: 4′-methyl-α-pyrrolidinohexanophenone and 4′-methyl-α-pyrrolidinobutyrophenone’, Forensic Science International 169 (1), pp. 32–42.
Westphal, F., Junge, T., Rösner, P., Sönnichsen, F. and Schuster, F. (2009) ‘Mass and NMR spectroscopic characterization of 3,4-methylenedioxypyrovalerone: A designer drug with α-pyrrolidinophenone structure’, Forensic Science International 190 (1), pp. 1–3.
Winstock, A. (2010) ‘Drugs survey’, Mixmag 14 January.
Winstock, A. R., Marsden, J. and Mitcheson, L. (2010) ‘What should be done about mephedrone?’, BMJ 340, p. c1605.
Wood, D., Davies, S., Puchnarewicz, M., et al. (2010) ‘Recreational use of mephedrone (4-methylmethcathinone, 4-MMC) with associated sympathomimetic toxicity’, Journal of Medical Toxicology, 1 April.
Wood, D. M., Davies, S., Puchnarewicz, M., et al. (2009) ‘Recreational use of 4-methylmethcathinone (4-MMC) presenting with sympathomimetic toxicity and confirmed by toxicological screening. Abstracts of the 2009 North American Congress of Clinical Toxicology Annual Meeting, September 21–26, 2009, San Antonio, Texas, USA’, Clinical Toxicology 47 (7), p. 733.
World Health Organization (2006) Assessment of khat (Catha edulis Forsk), 34th Meeting, Expert Committee on Drug Dependence. Available at: http://www.who.int/medicines/areas/quality_safety/4.4KhatCritReview.pdf.
Zaitsu, K., Katagi, M., Kamata, H., et al. (2009) ‘Determination of the metabolites of the new designer drugs bk-MBDB and bk-MDEA in human urine’, Forensic Science International 188 (1–3), pp. 131–139.
Zaitsu, K., Katagi, M., Kamata, H. T., Miki, A. and Tsuchihashi, H. (2008) ‘Discrimination and identification of regioisomeric β-keto analogues of 3,4-methylenedioxy-amphetamines by gas chromatography-mass spectrometry’, Forensic Toxicology 26 (2), pp. 45–51.














