Amphetamine drug profile

About amphetamine
A synthetic substance. Normally seen as a white powder, it acts as a stimulant of the central nervous system (CNS). It is believed that amphetamine was first manufactured in the 1880s by the German chemist Leuckart, although evidence for this is lacking. It appears that, as in the case of methamphetamine, systematic studies of its chemistry did not come about until the early twentieth century. Amphetamine has some limited therapeutic use, but most is manufactured in clandestine laboratories in Europe. It is under international control and closely related to methamphetamine.
Chemistry
Amphetamine (CAS-300-62-9) is a member of the phenethylamine family, which includes a range of substances that may be stimulants, entactogens or hallucinogens. Thus, amphetamine is α-methylphenethylamine.
According to IUPAC, the fully systematic name is α-methylbenzeneethanamine. The asymmetric α-carbon atom gives rise to two enantiomers. These two forms were previously called the [–]- or l-stereoisomer and the [+]- or d-stereoisomer, but in modern usage are defined as the R- and S-stereoisomers.
Molecular structure
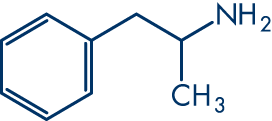
Molecular formula: C9H13N
Molecular weight: 135.2
Physical form
Amphetamine base is a colourless volatile oil insoluble in water. The most common salt is the sulfate (CAS-60-13-9): a white or off-white powder soluble in water. Illicit products mostly consist of powders. Tablets containing amphetamine may carry logos similar to those seen on MDMA and other ecstasy tablets.
Pharmacology
Amphetamine is a CNS stimulant that causes hypertension and tachycardia with feelings of increased confidence, sociability and energy. It suppresses appetite and fatigue and leads to insomnia. Following oral use, the effects usually start within 30 minutes and last for many hours. Later, users may feel irritable, restless, anxious, depressed and lethargic. It increases the activity of the noradrenaline and dopamine neurotransmitter systems. Amphetamine is less potent than methamphetamine, but in uncontrolled situations the effects are almost indistinguishable. The S-isomer has greater activity than the R-isomer. It is rapidly absorbed after oral administration. After a single oral dose of 10 mg, maximum plasma levels are around 0.02 mg/L. The plasma half-life varies from 4 to 12 hours and is dependent on the urinary pH: alkaline urine decreases the rate of elimination. A major metabolite is 1-phenyl-2-propanone, with smaller amounts of 4-hydroxyamphetamine. Analysis of amphetamine in urine is confounded because it is a metabolite of methamphetamine and certain medicinal products. Acute intoxication causes serious cardiovascular disturbances as well as behavioural problems that include agitation, confusion, paranoia, impulsivity and violence. Chronic use of amphetamine causes neurochemical and neuroanatomical changes. Dependence — as shown by increased tolerance — results in deficits in memory and in decision-making and verbal reasoning. Some of the symptoms resemble those of paranoid schizophrenia. These effects may outlast drug use, although often they resolve eventually. Injection of amphetamine carries the same viral infection hazards (e.g. HIV and hepatitis) as are found with other injectable drugs such as heroin. Fatalities directly attributed to amphetamine are rare. The estimated minimum lethal dose in non-addicted adults is 200 mg.
Synthesis and precursors
The most common route of synthesis is by the Leuckart method.This uses (P2P, BMK, phenylacetone) and reagents such as formic acid, ammonium formate or formamide to yield a racemic mixture of the R- and S-enantiomers. A much less common, but stereoselective, method is by reduction of the appropriate diastereoisomers of norephedrine or norpseudoephedrine. These precursors (1-phenyl-2-propanone, norephedrine and norpseudoephedrine) are listed in Table I of the United Nations 1988 Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances. The corresponding EU legislation is set out in Council Regulation (EEC) No 3677/90 (as later amended), which governs trade between the EU and third countries.
Mode of use
Amphetamine may be ingested, snorted and, less commonly, injected. Unlike the hydrochloride salt of methamphetamine, amphetamine sulfate is insufficiently volatile to be smoked. When ingested, a dose may vary from several tens to several hundreds of milligrams depending on the purity.
Other names
The term amfetamine (the International Non-Proprietary Name: INN) refers to a racemic mixture of the two enantiomers. Amfetamine is also the name required by Directives 65/65/EEC and 92/27/EEC for the labelling of medicinal products within the EU. Dexamfetamine is the INN for the (S)-α-methylbenzeneethanamine enantiomer also known as (+)-α-methylphenethylamine. Levamfetamine is the (R)-α-methylbenzeneethanamine enantiomer also known as (–)-α-methylphenethylamine. Other commonly used chemical names include 1-phenyl-2-aminopropane and phenyliospropylamine. Amphetamine is sometimes included with methamphetamine and other less common substances (e.g. benzphetamine) under the generic heading of ‘amphetamines’. Hundreds of other synonyms and proprietary names exist. ‘Street’ terms include speed, base and whizz.
Analysis
The Marquis field test produces an orange/brown coloration. The Simon test produces a red coloration that will distinguish amphetamine (a primary amine) from secondary amines such as methamphetamine (blue coloration). The mass spectrum shows little structure with a major ion at m/z = 44. Identification by gas chromatography–mass spectrometry can be improved by N-derivatisation, e.g. using carbon disulfide to form the isothiocyanate. Using gas chromatography, the limit of detection in urine is <10 μg/L.
Control status
The R- and S-enantiomers (levamfetamine and dexamfetamine respectively) as well as the racemate (a 50:50 mixture of the R- and S-stereoisomers) are listed in Schedule II of the United Nations 1971 Convention on Psychotropic Substances.
Medical use
Amphetamine has occasional therapeutic use in the treatment of narcolepsy and attention deficit hyperactivity disorder (ADHD).
Publications
Infographics and media
Bibliography
Iversen, L. (2006), Speed, Ecstasy, Ritalin: the Science of Amphetamines, Oxford University Press, Oxford.
King, L. A. and McDermott, S. (2004), ‘Drugs of abuse’, in: Moffat, A. C., Osselton, M. D. and Widdop, B. (eds.) Clarke's Analysis of Drugs and Poisons, 3rd edn, Vol. 1, pp. 37–52, Pharmaceutical Press, London.
Moffat, A. C., Osselton, M. D. and Widdop, B, (eds.) (2004), Clarke's Analysis of Drugs and Poisons, 3rd edn, Vol. 2, Pharmaceutical Press, London.
United Nations (2006), Multilingual Dictionary of Narcotic Drugs and Psychotropic Substances under International Control, United Nations, New York.
United Nations (2006), Recommended Methods for the Identification and Analysis of Amphetamine, Methamphetamine and their Ring-Substituted Analogues in Seized Materials (revised and updated), Manual for Use by National Drug Testing Laboratories, United Nations, New York.
United Nations Office on Drugs and Crime (2003), Ecstasy and Amphetamines Global Survey 2003, United Nations Office on Drugs and Crime, Vienna (http://www.unodc.org/pdf/publications/report_ats_2003-09-23_1.pdf).
United Nations Office on Drugs and Crime (2004), World Drug Report 2004, Vol. 1: Analysis, United Nations Office on Drugs and Crime, Vienna (http://www.unodc.org/pdf/WDR_2004/volume_1.pdf).



















